Key Takeaways
- Nutraceuticals are bridging the gap between food and medicine, offering science-backed support for immunity, metabolic health, cognition, and chronic disease prevention.
- The global nutraceuticals market is projected to reach $919 billion by 2030, driven by rising health awareness, personalized nutrition, and the demand for preventive care.
- Pharma companies are entering the nutraceutical space strategically, leveraging their R&D, compliance infrastructure, and healthcare networks to build credible, clinically validated Nutra brands.
- Consumers are shifting toward personalized, tech-driven wellness solutions, including DNA-based nutrition, microbiome-targeted probiotics, and AI-led supplement recommendations.
- Regulatory frameworks are tightening globally. In India, FSSAI governs most products, but CDSCO may soon oversee high-potency or therapeutic nutraceuticals.
- The future of nutraceuticals lies in integration: Rx drugs + Nutra + digital health in a unified care plan, powered by data and reimbursed by insurance.
- Trust is built through clinical evidence, transparent labeling, ethical sourcing, and certifications like USP, NSF, and EFSA-compliant approvals.
- Winning brands will be those that combine science with storytelling, compliance with convenience, and prevention with personalization.
I. Introduction – When Food Starts Acting Like Medicine
Last Updated: July 2025
A few years ago, a young woman named Ananya struggled with constant fatigue and recurring gut issues. Doctor visits offered temporary relief, but no long-term answers. Eventually, she turned to a mix of probiotics, omega-3 supplements, and vitamin D — suggested by a clinical dietitian, not a doctor. Within months, her energy returned, her digestion stabilized, and she felt more in control of her health than ever before.
Ananya's story isn't unique. It's part of a silent revolution happening around us — a global shift from reactive medicine to proactive health. And at the centre of this shift are nutraceuticals.
Coined by Dr. Stephen DeFelice in 1989, the term nutraceutical blends "nutrition" and "pharmaceutical." But the idea has been around far longer. Ancient systems like Ayurveda and Traditional Chinese Medicine were built around the concept that food can be your first medicine.

Today, the world is catching up.
From urban gyms to rural clinics, people are rethinking health not as the absence of disease but as the presence of vitality. They're adding turmeric capsules to their routines, sipping collagen-infused drinks, and popping magnesium gummies before bed — not out of trendiness, but because science is starting to back what tradition always knew.
This changing mindset is reshaping the healthcare industry. The global nutraceutical market crossed $590 billion in 2024, and it's growing fast — expected to near $920 billion by 2030. What started in wellness aisles has now entered boardrooms of major pharma companies. Abbott has Ensure. Bayer has Redoxon. GSK acquired Horlicks. Pfizer owns Centrum.
Why is pharma paying attention? Because nutraceuticals are no longer "alternative." They're becoming integral to the future of preventive healthcare.
In this guide, we'll explore how nutraceuticals are evolving — from pills and powders to AI-personalized plans. From grey regulatory zones to pharma-backed research labs. And from fringe to mainstream.
Let's uncover how nutraceuticals are rewriting the healthcare playbook — not with prescriptions, but with prevention.
II. Understanding Nutraceuticals – More Than Just Supplements on a Shelf
Back in 2020, Ramesh, a 52-year-old sales executive in Mumbai, had borderline cholesterol and frequent acidity. His doctor prescribed a statin but also suggested something unusual — "Try CoQ10 supplements and start your mornings with fortified oats."
That one suggestion changed how Ramesh viewed food. He didn't just eat to fill his stomach anymore. He started to eat to heal, energize, and protect.
This is the mindset nutraceuticals are designed for.
At their core, nutraceuticals are products derived from food sources that offer medical or health benefits — beyond basic nutrition. But they're not all the same. Let's break them down.
A. Types of Nutraceuticals
1. Dietary Supplements

These are the most familiar: vitamins, minerals, amino acids, and enzymes taken to address specific deficiencies or support health. Think of iron capsules, zinc tablets, or omega-3 soft gels.
2. Functional Foods

Everyday foods that have been enhanced with health-boosting ingredients.
Examples: probiotic curd, protein-enriched biscuits, or cereal fortified with B12.
3. Medicinal Foods

These are formulated specifically for patients under medical supervision, used in managing conditions like diabetes or renal disorders. Think: glucose control shakes or renal-specific protein powders.
4. Herbal Products

Inspired by traditional medicine systems, these include plant-based extracts, adaptogens, and ayurvedic formulations — like ashwagandha capsules or curcumin drops.
B. Forms & Delivery Methods
Gone are the days when supplements came only as pills. Today's consumer wants convenience and experience.
- Capsules & Tablets – Still the most common.

Traditional Format
- Powders – Especially in protein, energy, and gut-health mixes.

Versatile Mixing
- Gummies – A hit among both kids and adults for vitamins and melatonin.

Fun & Tasty
- Effervescent Tablets – Dissolve in water, often seen in energy and immunity products.

Quick Absorption
- Oral Dissolvable Strips – Rapid action with innovative delivery.

Instant Dissolve
- Transdermal Patches & Sprays – Gaining traction in global markets.
Advanced Delivery

Best Nutraceutical Supplements for Immunity Backed by Science
Read MoreC. How Nutraceuticals Differ from Pharmaceuticals
This distinction is important — nutraceuticals are not replacements for medicine, but they are increasingly recognized as a critical complement to healthcare.

Nutraceuticals vs Pharmaceuticals: Understanding the Divide and the Emerging Synergy
Read MoreA Quick Thought
Think of nutraceuticals like the preventive maintenance you do for your car. Pharmaceuticals are the mechanic you call when the engine fails. Both are essential — but wouldn't you rather prevent the breakdown?
III. Nutraceuticals in Preventive Medicine – The Shift from Sick Care to Self-Care
In early 2021, Priya, a 38-year-old schoolteacher in Delhi, was recovering from a mild COVID-19 infection. When her symptoms faded, she wasn't prescribed antibiotics — instead, her physician handed her a new routine: vitamin C + zinc effervescent tablets, a daily probiotic, and ashwagandha for fatigue.
For the first time, she felt like her doctor wasn't treating an illness — he was building her resilience.
This quiet revolution is playing out in clinics, homes, and pharmacies worldwide. People are no longer waiting to fall sick. They're taking health into their own hands. And nutraceuticals are leading this wave of preventive care.
A. From Cure to Prevention: A Global Health Shift
For decades, medicine focused on curing diseases. But today, preventing them is emerging as the smarter strategy — for patients, doctors, insurers, and governments.
Nutraceuticals are increasingly being used to:
- Boost immunity — Vitamin C, zinc, echinacea, elderberry, beta-glucans
- Support heart health — Omega-3, CoQ10, magnesium
- Strengthen bones & joints — Calcium, vitamin D3, glucosamine, collagen peptides
- Improve gut health — Probiotics, prebiotics, digestive enzymes
- Enhance cognitive performance — Ginkgo biloba, phosphatidylserine, B-complex
- Balance blood sugar & metabolism — Chromium picolinate, berberine, cinnamon extract
B. Immuno-Nutrition: The Post-Pandemic Awakening
The pandemic did more than just highlight immune health — it put immunity in every marketing headline.
But beyond buzzwords, the concept of immuno-nutrition has become a research-backed discipline. It explores how specific nutrients influence immune pathways. Clinical trials on ingredients like vitamin D, glutamine, and selenium have shown significant promise in modulating immune responses and reducing inflammation.
The result? A surge in demand for immunity blends, both in over-the-counter formats and hospital-prescribed medical nutrition.
C. Prevention for Chronic Conditions
Nutraceuticals aren't just about the flu or fatigue anymore. They're being studied — and in some cases prescribed — for long-term chronic conditions like:
- Type 2 Diabetes Nutrients like chromium, alpha-lipoic acid, and soluble fiber are part of emerging protocols.
- Arthritis Curcumin, collagen, and omega-3 are showing anti-inflammatory effects.
- Metabolic Syndrome Nutraceuticals help manage lipid profiles, insulin resistance, and inflammatory markers.
This shift isn't theoretical. Doctors, especially in integrative medicine, are now recommending nutraceuticals as part of prevention plans — often before a diagnosis is made.
D. Mental Wellness and the Gut-Brain Axis
One of the most exciting frontiers is the connection between the gut microbiome and mental health. Nutraceuticals that support gut health — like probiotics, L-glutamine, and prebiotic fibers — are being linked to reduced anxiety, better mood, and even improved cognitive performance.
As stress becomes a global health epidemic, adaptogens like ashwagandha and Rhodiola rosea are becoming staples in the nutraceutical toolkit.
In an age where people count steps, track sleep, and scan food labels — nutraceuticals aren't just a health choice, they're a lifestyle choice.
They're helping people stay well, not just get well.
IV. Pharma's Growing Interest in Nutraceuticals – When Big Medicine Bets on Wellness
When Bayer acquired the nutraceutical brand Care/of and Abbott rebranded Ensure into lifestyle-friendly formats, it wasn't just a marketing makeover — it was a signal.
A signal that pharma is no longer standing on the sidelines of wellness.
Pharmaceutical companies, known for their rigorous drug pipelines and billion-dollar R&D budgets, are now entering the nutraceutical space with surprising agility. What's changed?
A. A Business Case Backed by Trends
Pharma has always been driven by clear logic:
- High unmet need
- Market potential
- Long-term lifecycle value
And nutraceuticals check all three boxes:
B. Strategic Pharma Moves in Nutraceuticals
Here's how top pharma players are moving into Nutra — not cautiously, but confidently:
🔹 Pfizer
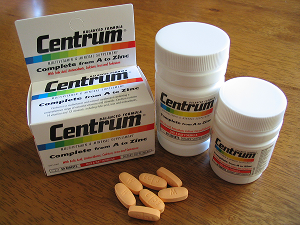
- Owns Centrum – a globally leading multivitamin brand.
- Also markets Caltrate (bone health) and Emergen-C (immunity support).
🔹 Bayer

- Expanded aggressively with Redoxon, Berocca, and the acquisition of personalized supplement brand Care/of.
- Focused on personalization, immunity, and women's health.
🔹 Abbott

- Transformed Ensure and Pediasure into lifestyle nutrition staples.
- Has a strong presence in clinical nutrition and therapeutic diet support.
🔹 GSK

- Owned Horlicks for decades (now under Hindustan Unilever).
- Invested in consumer health spin-offs and OTC nutrition portfolios.
🔹 Haleon (GSK spinoff)

- Owns Centrum and Emergen-C globally, exploring D2C personalization.
- Partnered with Hologram Sciences for AI-led nutrition personalization.
🔹 Otsuka (Japan)

- Through its U.S. company Pharmavite, acquired Bonafide Health for $425 million in 2023.
- Focused on women's health and hormone-support nutraceuticals.
C. Rx Meets Nutra: An Emerging Convergence
What used to be separate silos — prescription drugs vs over-the-counter supplements — are slowly blending.
Today's pharma companies are:
- Recommending nutraceuticals alongside drugs (e.g., CoQ10 with statins).
- Launching dual-path products that can work both OTC and under clinician supervision.
- Building hybrid research teams – nutritionists, formulation scientists, and pharma R&D experts.
D. Investment, Not Experimentation
Pharma's play in Nutra isn't casual. It's backed by:
- Dedicated innovation budgets for Nutra pipeline development
- Brand acquisitions to fast-track market access
- E-commerce arms for D2C scale (e.g., Haleon's online-first approach)
Story in a Snapshot
Imagine this: a 58-year-old man walks into a clinic. He leaves not just with a prescription for blood pressure meds, but a doctor-approved combo of magnesium, fish oil, and CoQ10 — all from the same pharma brand. That's the future pharma is designing — one where prevention and treatment sit side by side, and where science meets self-care.
That’s the future pharma is designing — one where prevention and treatment sit side by side, and where science meets self-care.
V. Manufacturing Landscape – Where Science, Safety, and Supplements Intersect
When Meera Shah, a QA head at a nutraceutical startup in Pune, visited a pharmaceutical plant for the first time, she was stunned. "It was like walking into a spaceship," she recalls. "The airlocks, the gowning protocol, the stainless steel — pharma doesn't leave room for dust, let alone doubt."
That day, she realized what separates nutraceutical manufacturing from pharmaceutical: it's not just what you make, but how rigorously you prove it's safe and consistent.
Yet, as demand for supplements skyrockets, nutraceutical manufacturers are stepping up — integrating pharma-grade standards, refining GMP practices, and adopting tech-enabled traceability.
Let's explore how the two industries compare — and where they're starting to overlap.
Nutraceutical vs Pharmaceutical Manufacturing: Key Differences
While pharma operates in a zero-error paradigm, nutraceuticals operate in a minimum-safe-error paradigm — but this is changing fast.
Building Trust: The Rise of Pharma-Grade Nutra Facilities
Modern Nutra plants are evolving with:
- Air-controlled cleanrooms
- In-line HPLC or NIR testing
- HEPA filtration
- Automated batching and packaging lines
- Fully traceable digital batch records
Many nutraceutical brands aiming for export markets or healthcare credibility are now voluntarily aligning with WHO-GMP or US-FDA norms, even though local regulations don't demand it.
Case Example:
A Bengaluru-based herbal brand, aiming to sell in Germany, was forced to upgrade its entire facility to match EFSA traceability norms — proving not just composition, but plant DNA purity, heavy metal absence, and microbiological safety.
The Grey Area: Regulatory Overlap and Consumer Confusion
In India, nutraceuticals are governed by FSSAI. But many products — especially those sold through doctors — walk the line between food and drug.
This leads to confusion:
- A Vitamin D3 capsule could be classified as a supplement or a drug, depending on dosage.
- An omega-3 product with EPA-DHA above 600mg may need pharma-level scrutiny.
The government is currently reviewing whether certain high-potency or condition-specific nutraceuticals should come under CDSCO oversight — similar to regulated "nutraceutical drugs" in Japan or the EU.
Expert Insight: Why Manufacturing Integrity Matters
"The biggest risk in nutraceuticals isn't efficacy. It's inconsistency. If one batch has 90% potency and the next has 60%, you lose trust and safety." — Dr. Neel Deshmukh, Head of Quality at a leading Indian contract Nutra manufacturer
Packaging: The Forgotten Link in Trust
Nutraceuticals are sensitive to:
- Light
- Moisture
- Heat
- Oxygen
Yet many Nutra brands still use packaging designed for appearance, not protection. That's changing with:
- Blister strips with desiccant backing
- Amber PET bottles with nitrogen flushing
- QR-coded packs for authenticity and usage guidance
A well-made supplement is invisible — no smell, no variation, no residue. That's what makes it hard to appreciate and easy to trust. But behind that trust is a manufacturing ecosystem that's evolving from wellness to evidence-backed excellence.
When pharma joins hands with Nutra in the factory, the product becomes more than just a supplement — it becomes a promise.
VI. Regulatory Environment – Who's Watching the Wellness Aisle?
In 2018, a Delhi-based startup launched a plant-based testosterone booster, claiming it could "triple stamina." Sales soared — until FSSAI stepped in. The product was banned for misleading claims and unapproved ingredients.
This isn't an isolated case. As the nutraceutical industry booms, regulation is playing catch-up. And unlike pharma, where CDSCO, FDA, and EMA operate with surgical precision, the world of nutraceuticals is a patchwork of evolving, and often confusing, frameworks.
Let's decode how this complex landscape is shaping up — in India, the US, and Europe.
India: FSSAI vs CDSCO – The Regulatory Tug of War
Nutraceuticals in India are currently regulated under:
FSSAI (Food Safety and Standards Authority of India)
- Governing Law: Food Safety and Standards Act, 2006
- Regulation: Covers dietary supplements, health supplements, functional foods, foods for special dietary use (FSDU), and medical purposes
- Scope: Defines permissible ingredients, dosages, labeling norms, and banned substances
- Key Notifications: FSS (Health Supplements, Nutraceuticals, Food for Special Dietary Use) Regulations, 2022
However, grey zones persist:
- Doctor-prescribed nutraceuticals sometimes carry Rx-style branding
- Some products exceed ingredient limits defined by FSSAI
- No mandatory pre-market approval (unlike drugs)
📌 Recent Development:
In early 2024, a government-appointed panel recommended bringing high-potency nutraceuticals and borderline products under CDSCO, India's drug regulator (source).
"It's no longer just food. We're looking at therapeutic use. It's time for clarity." — Senior FSSAI Official, on proposed regulatory reforms
USA: DSHEA and the Freedom-To-Sell Dilemma
The US follows a relatively liberal but structured approach.
- Regulatory Body: FDA (Food and Drug Administration)
- Governing Act: DSHEA (Dietary Supplement Health and Education Act), 1994
- Key Principle: Supplements are not pre-approved by FDA but must be safe and properly labeled
- Manufacturers are responsible for product safety, not the FDA
- Claims Allowed: Structure-function claims (e.g., "supports heart health"), not treatment claims
However:
- FDA can take action after the product is on the market if found unsafe or misleading
- Products like weight-loss pills and sexual enhancers are frequently flagged
📌 Trust Marker:
Third-party certifications like USP Verified, NSF, and Informed Choice help validate safety and potency for consumers.
European Union: The EFSA Gold Standard
Europe is strict — often the benchmark for global Nutra compliance.
- Regulatory Body: EFSA (European Food Safety Authority)
- Pre-market approval required for any health claim
- Scientific substantiation is mandatory (randomized trials, toxicity studies, dosage validation)
- Health claims are published in the EU Register of Nutrition and Health Claims
- Extensive ingredient list vetting under the Novel Food Regulation
📌 Example:
You cannot say "Vitamin D prevents infections" unless EFSA approves the language — the approved claim is "contributes to the normal function of the immune system."
What Global Brands Are Doing to Stay Safe
To navigate this regulatory maze, smart brands and pharma players are adopting:
- Multi-country compliance teams
- Dual-formulation strategy (low-dose for India, therapeutic dose for Rx-only markets)
- Legal-approved copywriting and claims teams
- Blockchain-backed ingredient traceability for export batches
Why Regulation Builds Trust
For consumers, regulation isn't red tape — it's a safety net.
Nutraceuticals are often self-prescribed. Without oversight, false claims, contaminated ingredients, or improper dosing can lead to real harm. That's why regulatory evolution is critical to the future of this industry.
"In pharma, you earn trust with trials. In nutraceuticals, you earn it with transparency." — Dr. Rajat Puri, Regulatory Affairs Consultant (Mumbai)
As nutraceuticals get closer to pharma in function, they must also come closer in accountability. Whether it's a probiotic sachet or a curcumin capsule, the message is clear:
If it acts like medicine, it must be made — and regulated — like medicine.
VII. Consumer Trends & Market Insights – From Wellness Niche to Everyday Norm
When Varun, a 29-year-old software developer in Bengaluru, began working 12-hour days, he didn't visit a doctor. Instead, he opened Instagram. Within minutes, he'd ordered an adaptogen-rich anti-stress drink, a melatonin gummy, and a probiotic for gut health — all based on influencer reviews and AI-generated recommendations.
This is the new face of nutraceutical consumption: tech-savvy, prevention-first, and deeply personalized.
Consumers across the globe — and particularly in India — are changing not just what they consume, but why and how they choose health products.
Let's decode the top shifts:
1. The Market is Not Just Growing. It's Surging.
- Global nutraceuticals market size (2024): ~$591 billion
- Forecast by 2030: ~$919 billion
- India's market: ₹450 billion (~$5.4 billion) and growing at ~21% CAGR, among the fastest globally
- Top contributors: Dietary supplements, functional foods, sports nutrition
Key Drivers:
- Urbanization and changing lifestyles
- Increased awareness post-COVID
- Higher disposable incomes
- E-commerce convenience and influencer ecosystems
2. Health Literacy is Powering Informed Choices
Today's consumers don't blindly trust celebrity-endorsed brands. They:
- Read labels for ingredient transparency
- Search clinical data before purchase
- Prefer plant-based, sugar-free, and non-GMO options
- Look for USP/NSF/AYUSH/FSSAI certifications
They're also asking tougher questions:
"Is this bioavailable?" "Is this patented?" "Is this backed by real science — or just trendiness?"
This shift is pushing brands to improve transparency, traceability, and education — not just marketing.
3. Wellness is the New Beauty
Beauty isn't just skin-deep anymore. It's gut-deep.
Welcome to "Beauty from Within" — a segment now exploding with:
- Collagen supplements for skin elasticity
- Biotin gummies for hair health
- Probiotic blends for acne and gut clarity
- Astaxanthin for skin glow and anti-aging
This convergence of skincare + nutrition is driving premium category growth, especially among women aged 25–45.
4. Personalized Nutrition is Here
One-size-fits-all doesn't cut it anymore. Brands are embracing:
- At-home gut microbiome testing
- Nutrigenomics kits
- AI-based quiz-to-product flows
📌 Example:
Brands like Care/of and BillionCheers offer customized vitamin plans based on quiz inputs, biomarkers, and even lifestyle analysis.
This isn't just marketing — it's a shift from shelf browsing to system-based personalization.
Regulatory Guide to Nutraceuticals in India: FSSAI vs CDSCO
Read More5. Sports Nutrition is No Longer Just for Athletes
The rise of fitness apps, smartwatches, and marathon culture has created a casual athlete class. They want:
- Pre-workout caffeine blends
- Plant-based protein powders
- Electrolyte sachets and BCAA drinks
- Creatine for strength and performance
In India, Tier 2 and Tier 3 cities are seeing a surge in gym-based supplement demand — especially among youth aged 18–30.
6. India's D2C Nutra Boom: Brands to Watch
The old model of pharma-distributed nutrition is now being challenged by content-first, millennial-focused brands. Some notable Indian examples:
What sets them apart?
- Direct-to-consumer channels
- Subscription models
- Social media-driven trust-building
- Minimal pharma branding — maximum lifestyle appeal
G. The Rise of Digital Shelf Influence
The "digital shelf" — Amazon, Flipkart, health marketplaces, and brand-owned stores — has become more important than pharmacy shelves.
- Consumers read verified reviews, not just labels
- Unboxing videos, influencer reels, and testimonial carousels sway decisions
- WhatsApp communities and Telegram channels discuss Nutra protocols like skincare or PCOS support
This means:
The new pharmacist might be… your favorite Instagram creator.
Nutraceuticals have evolved from being "doctor-advised" to "community-validated." The modern buyer isn't looking for cures. They're looking for control — over energy, mood, metabolism, skin, and sleep.
And that's exactly what this new wave of Nutra promises.
The brands that will win are not the ones shouting loudest — but those who listen deeply, personalize wisely, and deliver consistently.
VIII. Clinical Research & Evidence – From Grandma's Wisdom to Peer-Reviewed Journals
When a Mumbai-based startup launched a curcumin supplement in 2019, it went viral — not just because of its trendy packaging, but because it had something rare in the Indian Nutra space: a published clinical trial in the Journal of Dietary Supplements.
In an industry flooded with bold claims and borrowed science, this one brand stood out because it did the one thing most don't — it proved it.
Clinical evidence is no longer optional. It's becoming the currency of trust in nutraceuticals.
A. The Science Gap — and Why It's Closing Fast
Historically, nutraceuticals leaned on tradition. "Used in Ayurveda for 5,000 years" or "Backed by ancient Chinese medicine" worked on labels.
But today's consumers — and regulators — demand more:
- Randomized Controlled Trials (RCTs)
- Peer-reviewed publications
- Standardized ingredients
- Proven bioavailability
According to PubMed:
- Over 87,000 studies have been published on dietary supplements
- Nearly 14,000 are RCTs, the gold standard of evidence (Source: Nutraceuticals World, 2023)
B. What Clinical Research in Nutra Looks Like
Unlike pharma trials that may span 5–10 years, Nutra clinicals tend to be:
- Shorter duration (4 to 16 weeks)
- Smaller populations (30 to 200 subjects)
- Focused endpoints: inflammatory markers, energy levels, skin elasticity, sleep quality, etc.
- Placebo-controlled but not always double-blind
Example:
A 2022 trial on ashwagandha for anxiety measured cortisol reduction in 90 patients over 60 days — showing a 23% improvement over placebo.
Key Insight:
Most Nutra trials are nutritional interventions, not therapeutic claims — this limits what can be claimed on packaging but still offers value in building consumer trust.
C. Role of Real-World Evidence (RWE)
Given the preventive and lifestyle nature of nutraceuticals, Real-World Evidence (RWE) is emerging as a critical complement to trials.
Brands now track:
- Customer-reported outcomes (via apps, surveys)
- Wearable data (sleep, HRV, energy trends)
- AI-led feedback loops for formula optimization
📌 Example:
A D2C brand offering personalized sleep supplements uses post-purchase surveys and Fitbit integration to measure product efficacy over 30 nights.
D. Challenges in Nutra Research
Let's be honest — Nutra still faces trust barriers due to:
- Lack of funding for large-scale trials
- Variability in ingredient quality across brands
- Loose regulatory claim structures leading to pseudoscience
- Few trials conducted in Indian populations (still reliant on Western data)
E. Pharma is Raising the Bar
When pharmaceutical companies enter Nutra, they bring with them:
- Clinical trial infrastructure
- Regulatory-grade documentation
- In-house biostatisticians and pharmacologists
📌 Case Study:
Haleon (GSK's consumer health spinoff) runs formal studies on Centrum, Caltrate, and Emergen-C across multiple geographies — publishing results in open-access journals to build global credibility.
F. Certifications that Signal Trust
Since not all brands can afford clinical trials, certifications are becoming shorthand for credibility:
- USP Verified
- NSF Certified for Sport
- FSSAI + AYUSH (India)
- Informed Choice (sports nutrition)
- GRAS status (USA)
These validate:
- Ingredient sourcing
- Product potency
- Absence of banned substances
- Label accuracy
Final Thought
Science doesn't just validate ingredients — it elevates intention to evidence.
Nutraceuticals that are backed by trials, certifications, or transparent consumer data will win the long game — not because they claim to heal everything, but because they prove something real. In the age of smart consumers, honest science is the new branding.
In the age of smart consumers, honest science is the new branding.
IX. Challenges in the Nutraceutical Industry – The Bitter Aftertaste of a Booming Market
In 2022, a popular herbal testosterone booster was taken off shelves in India. Lab tests revealed it contained undeclared steroids. Its branding? "100% natural. Clinically validated."
This incident — one of many — shows why the nutraceutical space, despite its promise, is also grappling with credibility, compliance, and consumer confidence.
Behind the glossy labels and influencer reels lies a complex web of industry challenges — many of which can derail even the most promising brands if not addressed head-on.
A. Regulatory Grey Zones
In India and many parts of the world, nutraceuticals fall into a "neither food, nor drug" limbo:
- Products with medicinal claims bypass pharma laws under FSSAI
- Ingredient doses often exceed what's allowed in food, but aren't monitored like drugs
- No mandatory pre-market approval means almost anyone can launch a Nutra brand online
📌 Impact:
This confusion allows bad actors to flood the market, damaging trust even in compliant brands.
"If pharma needs a license to treat, Nutra should need a license to prevent." — Dr. K. Srinivasan, Regulatory Consultant
B. Lack of Standardization in Ingredients
Unlike pharma APIs, Nutra raw materials vary widely in:
- Purity
- Bioavailability
- Source (synthetic vs. natural)
- Absorption rate
Two "turmeric" supplements could have wildly different efficacies based on curcuminoid concentration or the presence of piperine.
📌 Example:
A 2021 lab comparison of Indian ashwagandha capsules found potency differences of over 300% between brands — none disclosed on labels.
C. Misinformation and Pseudo-Science
Marketing teams often outpace the science:
- "Boosts immunity by 400%" (no study cited)
- "Clinically proven" (based on rat studies)
- "Doctor recommended" (without disclosing affiliations)
In the absence of tight advertising controls, even well-meaning brands end up overselling outcomes.
💡 Result:
Erosion of consumer trust — especially among health-conscious, educated buyers.
D. Quality Control and Testing Gaps
Many small brands:
- Skip third-party batch testing
- Don't conduct stability tests for shelf life
- Fail to validate absence of heavy metals or microbial contaminants
And since most Nutra products are not sold under prescription, there's less post-market vigilance.
📌 In contrast:
Pharma batches go through 20+ checks before release. Nutra? Sometimes 3–5, if at all.
E. Sustainability and Ethical Sourcing
As plant-based ingredients grow in popularity, overharvesting and adulteration are becoming rampant:
- Shilajit mixed with charcoal
- Fake saffron in energy drinks
- Synthetic resveratrol passed off as "Japanese knotweed extract"
🌱 Challenge:
Building supply chains that are not just efficient — but ethical and traceable.
F. Pricing Pressure in a Crowded Market
With low entry barriers and heavy social media competition, brands resort to:
- Price wars
- Compromised quality
- Over-promise marketing
Margins shrink. Consumers suffer. Long-term trust erodes.
G. Lack of Trained Professionals
Unlike pharmacists or medical reps, nutraceutical advisors are rarely certified. Health stores and D2C brands often rely on:
- Social media creators
- Wellness coaches with no scientific training
- Chatbots built on basic FAQs
“Consumers ask: Can I take this with my thyroid meds? Many times, there’s no qualified person to answer.” — Dr. Shreya Agarwal, Clinical Nutritionist
The future of nutraceuticals is bright — but only if it's built on rigor, honesty, and responsibility.
In an industry that promotes vitality, the real measure of success isn't how fast a product sells — but how long it earns trust.
Brands that survive the next decade won't just be the boldest — they'll be the cleanest, clearest, and most clinically grounded.
X. The Rise of Tech-Driven Nutraceuticals – When Science Gets Personal
In a quiet corner of Bengaluru, a startup called GeneNutra mailed Anjali a cheek swab kit. Weeks later, she had a 12-page nutrition report based on her DNA — showing a high caffeine sensitivity, a low Vitamin D absorption gene, and a recommendation to avoid synthetic folate.
She didn't just get a supplement. She got a system. One built not for people like her, but for her.
This is the new frontier of nutraceuticals — where technology meets biology, and where products are no longer just manufactured — they're engineered around the individual.
A. Personalized Nutrition is Going Mainstream
Consumers don't want another multivitamin. They want to know:
- Why this supplement?
- Why this dose?
- Why now?
Tech is making this possible via:
- Nutrigenomics: Mapping how your genes affect nutrient needs
- Microbiome testing: Evaluating gut bacteria to guide probiotic/prebiotic use
- Blood biomarker kits: Measuring B12, Vitamin D, iron, and CRP from a finger-prick sample
- AI-based quizzes: Matching lifestyle habits with Nutra protocols
📌 Example:
Companies like Care/of (US), BillionCheers (India), and Persona (Nestlé) now offer data-led personalized supplement plans with monthly deliveries and dynamic formula adjustments.
B. Smart Formulation Powered by AI and Data Science
Nutraceutical R&D is being transformed by:
- AI algorithms analyzing clinical trials to predict synergistic ingredient blends
- Machine learning used to design delivery formats based on stability, bioavailability, and user preference
- Digital twins modeling individual nutrient response over time
📌 Real Use Case:
A global supplement brand uses AI to combine 10,000+ published studies to recommend ingredient stacks for brain health in aging adults — optimized by age, gender, and comorbidities.
C. App-Based Ecosystems and Habit Loops
Your next Nutra product won't come in a bottle — it may come with an app.
- Track your sleep, energy, digestion, skin health
- Get reminders, refill nudges, and even tele-nutrition consults
- Earn loyalty points for habit adherence or journaling
- Compare before-after biometrics from at-home tests
📌 Emerging Trend:
Nutra brands are becoming health platforms, offering not just pills, but personal dashboards for wellness tracking.
D. Delivery Innovation: Beyond Capsules
Today's consumers are bored of tablets. Enter:
- Oral dissolvable strips: Quick-release, tasty, travel-friendly
- Transdermal patches: For vitamins like B12 or magnesium
- Nano-emulsified sprays: For enhanced absorption of lipophilic nutrients
- Bioengineered gummies: Targeted nutrient release based on saliva enzymes
🧠 Benefit:
These formats boost compliance, absorption, and experience — the trifecta of effective supplementation.
E. Traceability and Transparency Through Blockchain & QR Codes
Some tech-forward brands now embed:
- QR codes linking to batch-specific CoAs (Certificates of Analysis)
- Blockchain trails to verify ingredient origin, test reports, and expiry
- Smart packaging that alerts users if products are stored improperly (temperature, humidity)
📌 Why it matters:
In an era of mistrust, transparency isn't a differentiator — it's an expectation.
F. Predictive Health + Supplementation as a Service
Imagine this:
- Your smartwatch notices declining HRV and sleep quality
- It syncs with your Nutra app
- The app recommends adaptogens + magnesium, and ships them automatically
- Next month, your dose adjusts as biomarkers improve
This isn't sci-fi. It's the future of "Supplementation as a Service" — already being piloted by startups in the US, Germany, and Singapore.
Tech is doing to nutraceuticals what wearables did to fitness: It's making it real, personal, measurable — and motivating.
The brands that lead this revolution won't just sell bottles. They'll build ecosystems that help people know themselves, trust what they consume, and feel the difference.
XI. The Future of Nutraceuticals in Pharma – From Side Aisle to Center Stage
Picture this.
It's 2030. You walk into a hospital for a routine check-up. The doctor doesn't just hand you a statin prescription — she prints out a dual path plan:
- Medication + lifestyle
- Personalized nutrition + preventive supplementation
- Covered by insurance, monitored via app, refillable via smart dispenser
And all of this is provided by a pharma + nutraceutical hybrid brand.
This is not a stretch — it's an emerging blueprint. Nutraceuticals are no longer a fringe wellness add-on. They are becoming pillars of modern, proactive healthcare.
Let's explore what this convergence could look like.
A. Pharma-Grade Nutraceuticals Will Become the Norm
As regulators tighten standards and consumers demand better quality, nutraceuticals will:
- Follow pharma manufacturing protocols (GMP, GLP)
- Use clinical-grade ingredients with standardized bioavailability
- Be prescribed in integrated treatment plans (e.g., curcumin with NSAIDs, probiotics with antibiotics)
📌 Future-ready brands will invest in:
- Clinical trial infrastructure
- R&D-backed health claims
- Physician outreach and detailing models
B. Insurance Will Begin to Cover Preventive Supplementation
Already, certain global insurers cover:
- Omega-3s for heart patients
- Vitamin D3 for osteoporosis management
- Medical nutrition for diabetes, IBD, or renal conditions
As cost-effective prevention gains traction, reimbursement for personalized supplements could become standard — especially for:
- Post-operative recovery
- High-risk patients (e.g., prediabetes, PCOS, cognitive decline)
- Preventive protocols in digital health plans
C. Rx + OTC + Nutra Will Merge Into One Ecosystem
The current divide between:
- Rx: Treat disease
- OTC: Manage symptoms
- Nutra: Support wellness
…will blur into a continuum of care. Pharma companies will offer full-spectrum portfolios, where a diabetes patient might be guided through:
- Metformin (Rx)
- Berberine or chromium supplements (Nutra)
- Glucometer & diet plan (Digital Health)
📌 This model:
Enhances patient adherence, improves clinical outcomes, and opens multi-channel revenue.
D. Pharma-Backed D2C Brands Will Go Global
Pharma isn't just about hospitals anymore. Future-facing companies will:
- Launch or acquire D2C Nutra brands
- Use AI, telemedicine, and apps to build consumer relationships
- Offer international SKUs with region-specific compliance
📌 Real trend:
Bayer's investment in Care/of, Nestlé's Persona, and India's own pharma houses building Nutra verticals like Zydus Wellness and Dr. Reddy's Rebalance.
E. Condition-Specific Nutra Will Be the Next Frontier
The future will move from generic multivitamins to precise condition-focused stacks like:
- Hormonal health blends for PCOS
- Brain performance kits for neurodegeneration
- Microbiome support for IBS
- Sleep + mood stack for burnout recovery
These clinically validated Nutra formulations will be:
- Recommended by doctors
- Tracked through apps
- Tuned based on biomarkers
📌 Why pharma is key:
Only pharma has the R&D, compliance, and HCP trust channels to scale this precision.
F. Global Regulatory Harmonization Will Raise the Bar
Today's Nutra landscape is fragmented — FSSAI, FDA, EFSA, TGA, CFDA — each with different rules. But by 2030, expect:
- Unified claims frameworks
- Cross-border ingredient approvals
- Global transparency dashboards for clinical data
Pharma players will lead this harmonization — creating a regulatory "highway" instead of regional backroads.
The next decade belongs to companies that don't treat pharma and nutrition as separate worlds, but as complementary missions:
- One treats.
- One prevents.
- Together, they empower.
The future of pharma won't just be prescription-driven. It will be prevention-powered — and nutraceuticals will be the foundation.
XII. Case Studies & Industry Voices – Who's Walking the Talk in Pharma + Nutraceuticals
While many brands talk about "science-backed wellness," a few are actually
investing, innovating, and influencing the Nutra space. These companies — from pharma giants to D2C disruptors — are not just selling supplements, they're
redefining how we access, trust, and experience preventive health.
Here are some stories worth paying attention to.
Case Study 1: Haleon (formerly part of GSK)

Global Pharma-Consumer Hybrid
What they did: Spun off from GSK in 2022, Haleon owns a powerhouse portfolio — including Centrum, Emergen-C, and Caltrate — and operates at the intersection of
clinical trust and consumer convenience.
Why it matters:
- Partnered with Hologram Sciences to bring AI-driven personalization into Nutra
- Uses real-world evidence dashboards to guide health claims
- Is integrating D2C, practitioner, and pharmacy modelsinto a seamless pipeline
"We are not a pharma company doing consumer health. We are a health company solving everyday prevention." — Haleon Executive, 2023 Investor Call
Case Study 2: Zydus Wellness (India)

Pharma Meets FMCG
What they did: Backed by Zydus Cadila (pharma), the brand owns Sugar Free, Complan, Nutralite, and Everyuth. It's now expanding into
doctor-advised Nutra (e.g., Zyceva for hair health, Revital H line extensions).
Why it matters:
- Uses pharma-grade facilities for over-the-counter Nutra
- Invests in clinical validation + market education
- Balances urban D2C growth with doctor-backed credibility
📌 Zydus represents
India's most mature pharma-backed Nutra portfolio — with presence across retail, hospitals, and digital.
Case Study 3: OZiva (India)

D2C Disruptor with Science Focus
What they did: Founded in 2016, OZiva became one of the first Indian brands to:
- Offer plant-based Nutra stacks
- Run in-house labs for product R&D
- Publish clinical validation summaries on its website
Hindustan Unilever acquired a majority stakeIn 2022,
📌 OZiva is an example of
tech-first, trust-led D2C that scaled with content, community, and clinical transparency.
Case Study 4: Pharmavite (Otsuka, Japan)

Clinical Depth with Lifestyle Reach
What they did: Pharmavite, maker of Nature Made, acquired
Bonafide Health in 2023 — a female hormone health Nutra brand.
Why it matters:
- Combined pharma-led R&D rigor with targeted wellness protocols
- Doubled down on menopause, fertility, and hormonal imbalance
- Adopted subscription-first business models
“We’re using pharma science to solve problems lifestyle brands are afraid to touch.” — Bonafide CEO, after acquisition
Voices from the Field
Here's what industry experts are saying:
"Pharma is no longer asking if they should enter Nutra. They're asking how fast they can scale it without losing scientific credibility." — Dr. Pranav Joshi, Global Strategy Lead, Nutraceutical Division, Bayer
"In my clinic, I now prescribe a statin and a CoQ10 capsule together. If pharma can offer both, from one trusted label — that's gold." — Dr. Meenakshi Rao, Cardiologist, Chennai
"Every Nutra claim needs either a study or a story. Ideally both." — Sameer Malhotra, Founder, NutraBridge Consulting
What These Cases Tell Us
- Integration is happening — pharma and Nutra are learning from each other
- Personalization is key — from DNA kits to tailored routines
- Proof is non-negotiable — real-world validation is now a competitive advantage
- Brand trust > celebrity hype — especially in preventive health
XIII. FAQs – Answers to Your Top Questions About Nutraceuticals in Pharma
What is the difference between nutraceuticals and pharmaceuticals?
Nutraceuticals are food-based products designed to support health or prevent disease. Pharmaceuticals are drugs that diagnose, treat, or cure specific medical conditions and undergo strict clinical trials and regulatory approval.
📌 Example:
- Vitamin D3 in 400 IU = Nutraceutical
- Vitamin D3 in 60,000 IU (Rx) = Pharmaceutical drug
Are nutraceuticals regulated like drugs?
Not exactly.
- In India, nutraceuticals are regulated by FSSAI as food products — not by CDSCO unless they cross dosage thresholds.
- In the US, they fall under DSHEA (1994) — supplements are not pre-approved by the FDA.
- In Europe, EFSA mandates pre-market health claim approval and strict scientific backing.
Do nutraceuticals actually work?
Many do — but only if they're science-backed, properly dosed, and taken consistently. Products with clinical validation, third-party testing (e.g., USP, NSF), and transparent labeling are far more trustworthy than unverified, hype-driven supplements.
Can nutraceuticals replace medicines?
No. Nutraceuticals are designed to support or prevent — not treat.
They may:
- Complement existing therapies (e.g., probiotics with antibiotics)
- Help maintain health (e.g., omega-3 for heart support)
But they should not replace prescribed drugs without medical advice.
Are there side effects of taking nutraceuticals?
Yes — if misused.
- Overdosing on fat-soluble vitamins (like A, D, E, K)
- Allergic reactions to herbs or fillers
- Drug-nutra interactions (e.g., St. John's Wort can reduce drug efficacy)
Always consult a healthcare provider before starting a new supplement routine.
How do I know if a supplement is trustworthy?
Check for:
- Certifications (FSSAI, USP, NSF, Informed Choice)
- Ingredient transparency (standardized extracts, declared potency)
- Clinical support (mention of published trials or human studies)
- Batch traceability or QR scan codes
Avoid brands that promise instant results or make unproven medical claims.
Are pharma companies really serious about nutraceuticals?
Absolutely. Leading pharma players like Pfizer, Bayer, Abbott, Haleon, and Zydus are investing heavily in nutraceuticals — through:
- Acquisitions
- R&D-backed Nutra lines
- Personalized supplement platforms
This is a strategic expansion, not a side hustle.
What's the future of nutraceuticals?
- Personalized and tech-enabled supplementation
- Condition-specific formulations with clinical data
- Insurance and Rx integration in chronic disease prevention
- Regulatory harmonization across regions
Nutraceuticals will become an integral layer of modern healthcare — especially in aging, metabolic health, and mental wellness.
