by Simantini Singh Deo
8 minutes
People As The Largest Risk Vector – CCS Approaches To Human Factors
Human error is pharma’s biggest risk. Learn CCS strategies to manage personnel factors, reduce contamination, and improve compliance.
In pharmaceutical manufacturing and microbiology, human error remains one of the most significant sources of risk. Despite advanced automation, rigorous procedures, and robust quality management systems, people remain the largest risk vector in contamination, deviations, and regulatory non-compliance.
Operators, laboratory personnel, and support staff can unintentionally introduce errors through lapses in aseptic technique, incorrect sampling, or failure to follow established protocols. Understanding human factors and implementing strategies to mitigate these risks is therefore critical to maintaining product quality, ensuring patient safety, and preventing regulatory issues such as FDA Form 483 observations or warning letters.
Contamination Control Strategies (CCS) emphasize systematically managing human-related risks alongside equipment, processes, and environmental controls. Human factors in this context refer to the interactions between personnel, equipment, procedures, and the environment that influence the likelihood of errors or contamination events. By recognizing and addressing these factors, pharmaceutical organizations can reduce the potential for human error and strengthen overall contamination control programs.
Understanding Human Factors In Pharmaceutical Manufacturing
Human factors in pharmaceutical operations encompass a wide range of influences, including knowledge, skills, behavior, workload, fatigue, ergonomics, and cognitive biases. Errors are often unintentional and occur not because personnel are careless, but due to systemic weaknesses, inadequate training, or process complexity. For example, a worker may inadvertently touch a critical surface in a cleanroom because procedures are unclear, or because the workflow is inefficient and requires excessive hand movements.
Regulators, including the FDA and EMA, increasingly recognize that addressing human factors proactively is as important as controlling equipment or environmental risks. An inspection might reveal repeated deviations, improper gowning, or environmental excursions, and auditors often investigate whether these issues were caused by systemic gaps or individual operator errors. A well-implemented CCS approach treats human factors as a critical part of contamination risk management, integrating personnel behavior into broader quality systems.
The Role Of Training And Competency
One of the primary tools for managing human-related risk is comprehensive training and competency assessment. Personnel must be trained not only on standard operating procedures (SOPs) but also on the rationale behind each step, including aseptic technique, gowning protocols, cleaning procedures, and environmental monitoring. Understanding the “why” behind procedures significantly improves adherence and reduces errors caused by routine tasks being performed mechanically.
Competency assessments should be ongoing and objective. Techniques such as direct observation, practical testing, simulated contamination events, and periodic retraining help ensure personnel maintain high levels of skill. For instance, operators in a sterile filling area may be evaluated on their gowning technique, manipulations within isolators, and ability to perform cleaning and disinfection without introducing contamination. Auditors often scrutinize training records to ensure that staff involved in critical operations were adequately trained and competent at the time of inspection.
Continuous training also helps address knowledge decay, which occurs when personnel forget key steps over time. By scheduling periodic refresher training and competency evaluations, organizations maintain operational readiness and reduce the likelihood of human error.
The Role Of Training And Competency
One of the primary tools for managing human-related risk is comprehensive training and competency assessment. Personnel must be trained not only on standard operating procedures (SOPs) but also on the rationale behind each step, including aseptic technique, gowning protocols, cleaning procedures, and environmental monitoring. Understanding the “why” behind procedures significantly improves adherence and reduces errors caused by routine tasks being performed mechanically.
Competency assessments should be ongoing and objective. Techniques such as direct observation, practical testing, simulated contamination events, and periodic retraining help ensure personnel maintain high levels of skill. For instance, operators in a sterile filling area may be evaluated on their gowning technique, manipulations within isolators, and ability to perform cleaning and disinfection without introducing contamination. Auditors often scrutinize training records to ensure that staff involved in critical operations were adequately trained and competent at the time of inspection.
Continuous training also helps address knowledge decay, which occurs when personnel forget key steps over time. By scheduling periodic refresher training and competency evaluations, organizations maintain operational readiness and reduce the likelihood of human error.
Designing Workflows To Reduce Human Error
CCS emphasizes that processes should be designed with human factors in mind to reduce the likelihood of mistakes. Workflow design includes ergonomic layout, clear labeling, logical step sequencing, minimizing manual interventions in critical areas, and reducing unnecessary complexity. Poorly designed workflows can inadvertently increase contamination risk even for highly trained personnel.
For example, a poorly arranged cleanroom may require operators to cross traffic paths or reach over critical equipment, increasing the risk of contamination. A well-designed workflow minimizes hand movements, reduces opportunities for cross-contamination, and ensures that operators can perform tasks efficiently and correctly. Visual cues, such as floor markings, signs, or color-coded zones, help guide personnel behavior and reinforce SOP adherence.
Automation and digital tools can also complement workflow design by reducing reliance on human action for critical steps. Automated filling systems, barcode verification, and real-time environmental monitoring alerts help maintain process integrity while minimizing human error. However, technology is not a replacement for training and awareness—operators must understand how automated systems function and their role in contamination control.
Monitoring And Assessing Human Performance
Monitoring human performance is a vital component of a CCS approach. Observing operators in real time, collecting data from environmental monitoring, reviewing deviations, and analyzing batch records can highlight patterns linked to human factors. Identifying areas where errors occur most frequently allows organizations to implement targeted improvements.
For instance, if microbial excursions consistently occur during a specific shift, investigation may reveal that operators during that shift are not following gowning procedures correctly or that environmental monitoring is not performed as scheduled. Addressing these issues might include retraining, workflow modifications, or increased supervision.
Auditors pay close attention to how human performance is monitored. Evidence of regular observation, coaching, and performance assessment demonstrates a proactive approach to minimizing human error. Facilities that lack consistent oversight of human factors are more likely to receive Form 483 observations or warning letters.
SOP Design And Usability
Standard operating procedures are central to minimizing human error, but their effectiveness depends on clarity, conciseness, and usability. Complex or poorly written SOPs increase cognitive load and can lead to mistakes. CCS approaches advocate designing SOPs that are intuitive, with clear step-by-step instructions, visual aids, and checklists where appropriate.
Human factors considerations also include language, terminology, and comprehension. SOPs should be tested with operators to ensure they are practical and easily understood. During audits, regulators often review whether SOPs are followed consistently and whether they are usable in real-world operations. SOP usability can be enhanced by including diagrams of critical steps, highlighting risk points, and providing rationale for each action to reinforce correct behavior.
Human Factors In Deviation And OOS Investigations
Deviations and microbial out-of-specification (OOS) results often reveal the influence of human error. CCS approaches emphasize considering human factors as potential contributors during investigations. This includes reviewing operator actions, training records, adherence to SOPs, and environmental conditions at the time of the incident.
For example, an OOS in a sterile injectable may occur because an operator inadvertently touched a critical surface or failed to follow gowning procedures. Investigations should determine whether the error was isolated or systemic, leading to targeted corrective and preventive actions (CAPA). Addressing human factors in root cause analysis strengthens audit defensibility and demonstrates that the organization proactively mitigates contamination risks.
Leveraging Technology To Mitigate Human Risk
Technology can play a significant role in reducing human-related risks. Automation, digital checklists, environmental monitoring sensors, and real-time alert systems minimize manual interventions and improve data accuracy. For example, automated filling lines reduce the number of hand manipulations required, while electronic records and monitoring systems ensure that deviations are immediately flagged.
CCS approaches advocate human-technology integration, where operators are trained to understand and interact effectively with automated systems. Auditors often assess whether personnel can competently operate and respond to technology-driven alerts, ensuring that technology supports rather than replaces human vigilance.
Culture Of Quality And Accountability
Managing human factors effectively requires a culture of quality and accountability. A strong quality culture encourages personnel to follow procedures meticulously, report deviations without fear, and participate in continuous improvement initiatives. Leadership plays a key role in reinforcing this culture, demonstrating that quality and safety are priorities, and recognizing staff for adherence to best practices.
Auditors often assess organizational culture indirectly through interviews, observations, and documentation review. Companies that demonstrate accountability, transparency, and proactive contamination control are less likely to receive 483 observations related to human error. Encouraging a culture of quality helps personnel understand that their actions directly impact product safety and regulatory compliance.
Real-World Examples Of Human Factors In Contamination
Several real-world cases highlight how human factors contribute to contamination risk:
- Improper Aseptic Technique: In one sterile injectable facility, repeated microbial excursions were traced to operators touching critical surfaces during filling. Retraining and workflow adjustments eliminated the excursions and demonstrated the importance of human behavior monitoring.
- Gowning Deviations: A cleanroom OOS investigation revealed that operators frequently skipped glove changes between tasks. CAPA included retraining, SOP clarification, and environmental monitoring trend analysis.
- Mislabeling & Documentation Errors: Errors in labeling and batch records, often caused by fatigue or distraction, led to deviations in product release. Implementing double-check systems and ergonomic workflow design minimized human error.
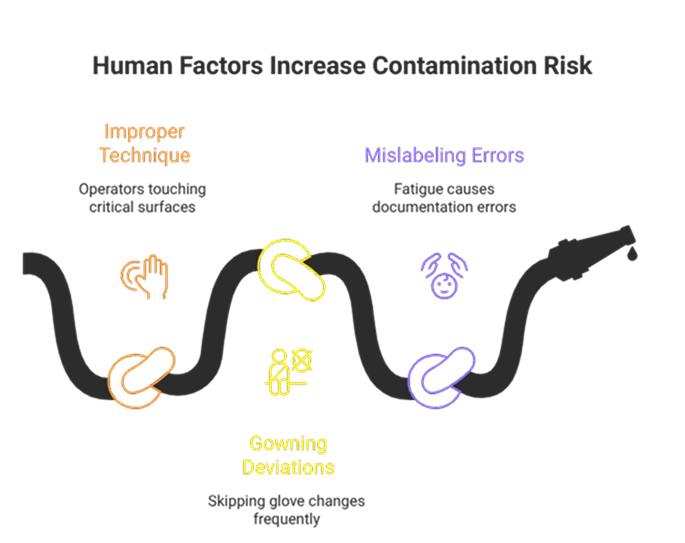
These examples illustrate that even well-trained personnel can become vectors for contamination if processes and human factors are not properly managed!
Integrating Human Factors Into CCS
A contamination control strategy that addresses human factors must integrate personnel considerations across all elements of the production and laboratory environment. This includes:
- Incorporating ergonomic and workflow design principles.
- Ensuring SOPs are clear, concise, and tested for usability.
- Implementing comprehensive training and ongoing competency assessments.
- Monitoring performance through environmental data, deviations, and audits.
- Leveraging technology to reduce manual interventions.
- Promoting a culture of quality and accountability.
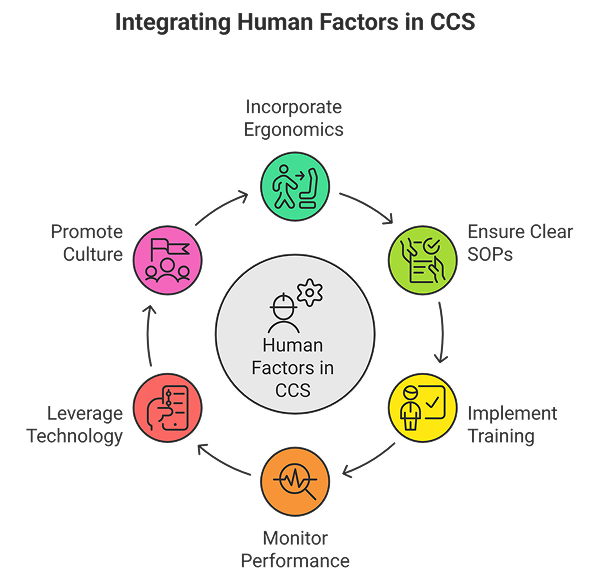
By considering human factors as part of a holistic CCS, organizations reduce contamination risk, improve compliance, and strengthen audit readiness.
Audit Readiness And Regulatory Expectations
Auditors focus heavily on human factors during inspections. They evaluate training records, competency assessments, SOP adherence, workflow design, and how deviations are investigated. Organizations that proactively address human factors demonstrate that contamination control is systematic, science-based, and preventive rather than reactive.
Regulators expect evidence that human-related risks are identified, analyzed, and mitigated as part of a broader quality system. Documentation, observation records, and CAPA effectiveness are key elements in demonstrating that human factors are managed appropriately. A robust CCS approach ensures that audits are passed with minimal observations and reinforces regulatory confidence.
In Conclusion
People are the largest risk vector in pharmaceutical microbiology and manufacturing. Even with advanced equipment, strict SOPs, and rigorous quality systems, human error can introduce contamination and deviations if human factors are not proactively managed. CCS approaches provide a structured framework to address these risks, integrating training, competency, workflow design, SOP usability, performance monitoring, technology, and organizational culture.
By understanding and mitigating human factors, organizations reduce regulatory risk, enhance product quality, and protect patient safety. A robust contamination control strategy recognizes the critical role of personnel, ensuring that people contribute to quality and safety rather than becoming the largest risk vector. With a strong focus on human factors, pharmaceutical companies can build resilient, audit-ready systems that demonstrate excellence in both compliance and operational performance.