by Vaibhavi M.
6 minutes
Branded, Generic, or Biosimilar Drugs? Breaking Down The Basics Of Pharma
Understand branded, generic, and biosimilar drugs, their differences, safety, costs, and FDA approval for smarter healthcare decisions.
Ever stared at your prescription’s bill and thought, “Why does this cost a fortune?” You’re not alone! I recently bumped into a friend of mine on the way back home from a chemist, coincidentally, both had the same medicine, BUT! At a different cost instead, I had the same citerizine combination tablet at a quarter of the price as my friend. Yes! You guessed it right, I took a smarter option, the GENERIC VERSION.
Ever wondered why two pills with the same ingredients have different prices? The pharma world is packed with buzzwords like branded, generic, biologics and biosimilar, but what does that mean? Are generics less effective? Are Biosimilars not safe? Are branded medicines always better?
Let's break it down. Whether you’re a curious patient, a healthcare pro, or just someone tired of expensive meds, this blog is your ultimate bucket of pharma’s most misunderstood labels. Let’s get to know what branded, generic, and biosimilar are. The development process & patents also bust some myths, and help you become a more competent healthcare decision-maker!
What Are Branded Medicines?
A branded medicine, or a non-generic drug, is a medication marketed under a proprietary, trademark-protected name by a pharmaceutical company. This name is distinct from the International Nonproprietary Name (INN).

Development process of branded drugs
The manufacturing company starts by researching and developing a new chemical to treat the disease. It further includes the New Drug Application (NDA) and Investigational New Drug Application (IND) after all the FDA procedures, the drug substance is forwarded for manufacture under regulatory compliance.
Now that the development process is the same as the other versions of drugs, why are branded medicines expensive? - The answer is the manufacturing cost and patents. New brand-name drugs are usually protected by patents prohibiting others from selling the same drug's generic versions.
The company manufacturing branded medicines has invested a large sum in the development process. It has invested significant funds in research, clinical trials, FDA applications, manufacturing, packing, transporting, and marketing. After all this process, a patent plays a crucial role. These patents give them the right to manufacture, distribute, export and import their inventions for about twenty years.
The brand-name medication receives initial approval as a patented drug. The expiration of a brand-name medication patent enables other companies and manufacturers to legally make generic versions of that medication and biosimilar drug forms.
What Are Generic Medicines? - The Affordable Twins
A million-dollar question: What is the difference between generic and branded medicine? Are generic medicines safe? Let’s break the myths.
Generic medicines are nothing but the branded medicine in the International Nonproprietary Name (INN). The generic medicine comes into existence after the expiry of the patent filed by the company that manufactures branded medicines. These medicines contain the same active pharmaceutical ingredients (API), excipients, concentrations and dosage forms and are manufactured to the same safety and quality standards. To provide equal effectiveness, strength, stability, and efficacy.
The FDA states that generic drugs must demonstrate equivalent performance compared to patent medicines. Generic drugs require mechanisms identical to the original medications, the same strength and safety profile, therapeutic index, route of administration, stability, and quality to pass the FDA approval. Generics are just as effective as the branded medicines. They may look different in shape, colour, and packaging, but they work the same in your body.
What is the role of the FDA in bringing generic drugs to market? Like brand-name drugs, generic medications undergo an equally rigorous approval process. Well, instead of filling various applications, the generic manufacturer is needed to fill an Abbreviated New Drug Application (ANDA). The FDA's Center for Drug Evaluation and Research, Office of Generic Drugs, receives an Abbreviated New Drug Application (ANDA), which comprises information necessary for a generic drug product's evaluation and eventual approval. Applications for generic drugs are referred to as "abbreviated" since they typically do not need to provide preclinical (animal) and clinical (human) evidence to prove efficacy and safety. Rather, a generic applicant needs to provide scientific proof that their medication is bioequivalent, meaning it functions similarly to the innovator drug.
Why are generic medicines cheaper? The simple answer is that they are less expensive because they don’t bear the cost of research, development, clinical trials, and marketing.
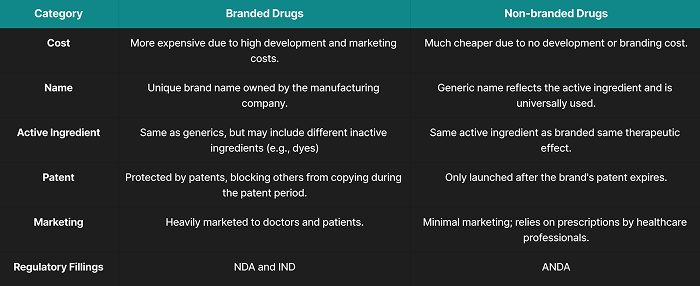
Difference Between Branded And Generic Medicines
Biosimilars: The Biotech Game-Changers
To understand biosimilars, we first need to understand Biologics. Biologics or Biological products are advanced medicines made from living cells or organisms. They include vaccines, blood components, gene therapies, and therapeutic proteins. Unlike conventional drugs made from chemical synthesis, biologics are often made using modern biotechnology and can contain proteins, sugars, or nucleic acids sourced from humans, animals, or microbes. Biologics are used to treat complex conditions such as cancer, auto-immune disease, especially those with limited or no existing treatments.
Now, what are biosimilars? As the name suggests, BIO-SIMILAR is similar to the biological product. Unlike chemically synthesised generic drugs, replicas of branded medicine, Biosimilars are biologics, made from living cells like plants or animals. Because of their complex nature, biologics can't be copied exactly like chemical drugs. So, while biosimilars aren’t identical to the original biologic, they’re highly similar with no meaningful differences in safety, strength, or effectiveness. Minor variations between batches are normal due to the nature of living systems, like yeast, bacteria, and animal cells. Considering the sources of these medications, there is some inherent variability, but they don’t affect the drug's therapeutic index. Biosimilars offer more affordable options for advanced treatments.
Biosimilars can be unofficially called the generic version of biological products. As the biological product undergoes a regulatory process called a Biologics Licensing Application(BLA), the biosimilars also undergo a regulatory process, like an abbreviated version. The FDA’s Centre for Biologics Evaluation and Research (CBER) usually reviews and approves new biologics and biosimilars.
Common Myths Busted - And Why You Shouldn’t Fall for Them
Let’s clarify false interpretations of some of the most common misconceptions about branded, generic, and biosimilar drugs.

“Generics are weaker or less effective.”
False- Generics must prove bioequivalence to the branded version, meaning they work the same way in your body and deliver the same clinical effect. The FDA doesn’t sacrifice quality here; if approved, it’s effective.
“Biosimilars are unsafe because they’re not exact copies.”
Wrong again- Biosimilars are held to high standards. While they're not identical because biologics are made from living cells, they are highly similar in structure and function and show no clinical differences in safety, purity, or potency.
“Branded drugs are always better.”
Not necessarily- What you’re often paying extra for is brand recognition, not performance. Once patents expire, generics and biosimilars step in with the same effectiveness, minus the high cost and commercials.
“Switching to a generic or biosimilar could harm my treatment.”
Unlikely, and here’s why- Pharmacists and doctors monitor these transitions closely. Generic substitutions are often automatic, and biosimilars undergo rigorous studies to prove they perform the same clinically as their branded products.
Conclusion
Today, understanding the difference between branded, generic, and biosimilar drugs can make a huge difference for your health and wallet. Branded medicines often lead the way, but generic drugs and biosimilar medicines provide safe, affordable alternatives after patent expiration.
Don’t let pharma myths or branding influence your choices. Whether you're dealing with chronic illness or a one-time prescription, knowing your medication options helps you make cost-effective decisions. Always consult your doctor or pharmacist for available generic or biosimilar alternatives because in the world of pharma, smart choices mean better care and big savings.




