by Ravindra Warang
7 minutes
What is Chromatography? A Beginner's Guide with Examples
Explore chromatography basics, types, and applications in pharma, food, and forensics with clear examples and key principles explained.
One evening, Dr. Meera, a pharmaceutical scientist, sipped her favorite hibiscus tea after a long day in the lab. As crimson streaks unfurled into the hot water, she smiled. That simple act—tea infusing water—mirrored something much deeper: the science of chromatography. What seemed like art in a teacup was, in fact, the core principle she relied on daily to analyze critical drug samples.
Chromatography may sound complex, but its core is something we see all around us. From forensic science to clinical labs and food testing to pharmaceuticals, it plays an invisible yet essential role in protecting human health and scientific integrity.
What is Chromatography?
Chromatography is a laboratory technique used to separate, identify, and quantify the individual components of a mixture. It helps scientists analyze compounds in pharmaceuticals, foods, environmental samples, and biological fluids.
The word “chromatography” is derived from the Greek words chroma (color) and graphein (to write). It was coined by Russian botanist Mikhail Tsvet in 1903, who separated plant pigments on a column and watched colored bands appear—hence the name.
While early chromatography separated visible pigments, modern applications deal with invisible molecules. Chromatography has become a vital tool in the pharmaceutical industry, where it ensures that drugs are pure, stable, and safe.
The Principle Behind Chromatography
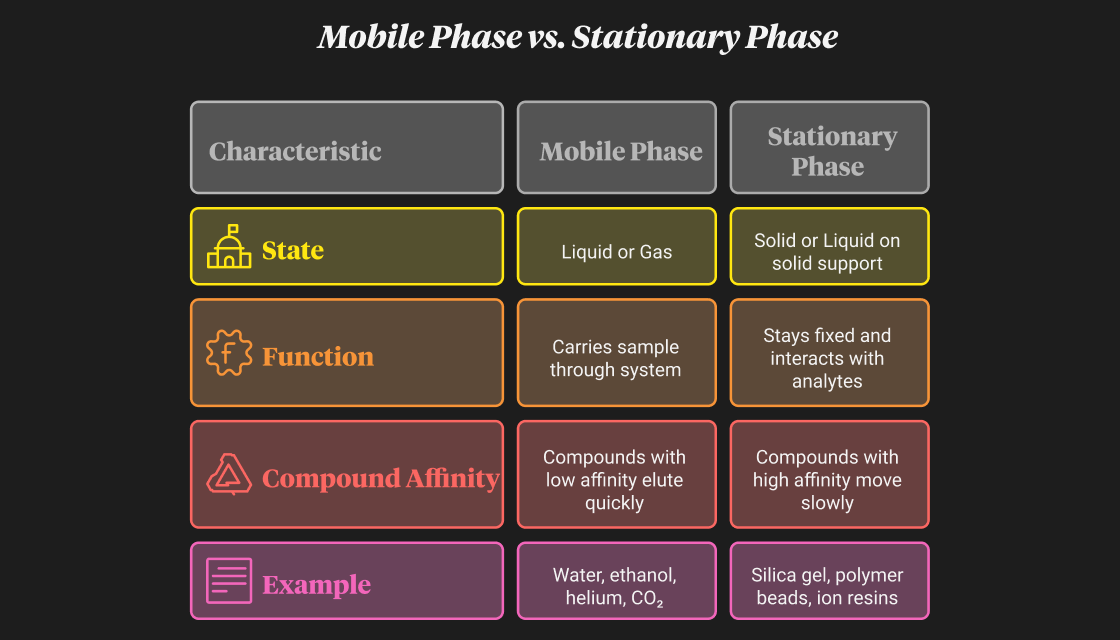
At its core, chromatography works on the principle of separation by differential affinity. When a mixture moves through a system composed of a mobile phase and a stationary phase, each compound interacts differently with these two phases. The mobile phase carries the sample forward, while the stationary phase holds on to some components more than others. Those that interact more with the stationary phase move slower; those that prefer the mobile phase move faster.
This difference in interaction causes the components of the mixture to separate as they travel. The amount of time each compound takes to pass through the system is called its retention time. This principle allows scientists to identify and measure each compound based on how long it stays in the system. A detector then records this information as a series of peaks on a chromatogram.
There are several types of interactions that determine how components separate:
- Adsorption (surface interaction): Common in Thin-Layer Chromatography (TLC)
- Partitioning (solubility-based): Seen in Liquid-Liquid systems
- Ion exchange (charge-based): Used in separating ions or charged molecules
- Size exclusion (molecular size-based): Used in protein and polymer analysis
- Affinity (biological recognition): Ideal for purifying enzymes or antibodies
The effectiveness of this separation depends on factors such as the polarity of compounds, column material, mobile phase composition, and flow rate. Even small changes in temperature or pH can affect how compounds interact with the system.
To simplify the concept, here’s a comparison table:
Types of Chromatography
Chromatography isn’t a single method—it’s a family of techniques tailored to different kinds of samples and separation goals. Some methods use liquids, others use gases. Some operate in simple labs, while others require high-end instrumentation.
The core principle remains the same: components of a mixture move at different speeds based on their interactions with the mobile and stationary phases. Let’s look at the most common types you’ll encounter.
Paper Chromatography
This is one of the oldest and simplest forms of chromatography. Here, a strip of filter paper acts as the stationary phase, and a liquid solvent (like water or alcohol) is the mobile phase.
As the solvent moves upward by capillary action, it carries sample components at different speeds. It’s commonly used in educational settings or to analyze food colors, inks, and plant pigments.
Thin-Layer Chromatography (TLC)
TLC works similarly to paper chromatography but uses a thin layer of adsorbent material—like silica or alumina—coated on a glass or plastic plate. The sample is spotted near the base, and the plate is dipped in solvent. As the solvent rises, it separates the compounds.
TLC is often used in pharma labs for checking the progress of chemical reactions or identifying unknown substances.
Gas Chromatography (GC)
GC is used for separating volatile substances. A gas (like helium) acts as the mobile phase and flows through a narrow column coated with a liquid stationary phase. The sample is vaporized and carried by the gas through the column, where its components separate based on their boiling points and polarity.
It’s commonly used to detect residual solvents in drug formulations, or in forensic labs to analyze blood alcohol levels.
High-Performance Liquid Chromatography (HPLC)
HPLC uses high-pressure pumps to push liquid mobile phase through a tightly packed column. The stationary phase is usually made of silica particles chemically modified with hydrophobic groups like C18. HPLC offers high resolution and is the most widely used technique in pharmaceutical analysis—for APIs, impurities, stability testing, and more.
Ultra-High Performance Liquid Chromatography (UHPLC)
UHPLC is an advanced version of HPLC. It uses smaller particle sizes (<2 µm) and operates at higher pressures (up to 15,000 psi). This leads to faster separations, better resolution, and shorter analysis times—making it ideal for high-throughput labs.
It’s especially useful in regulated pharma environments, where speed and reproducibility are critical.
Size-Exclusion Chromatography (SEC)
Also known as gel filtration, SEC separates molecules based on size. Large molecules bypass the porous stationary phase and elute first, while smaller ones enter the pores and take longer. It’s widely used for analyzing protein aggregates, antibodies, and polymer molecular weights.
Ion Exchange Chromatography (IEC)
IEC separates molecules based on charge. Positively or negatively charged groups are attached to the stationary phase, which binds to oppositely charged analytes. By gradually changing the pH or salt concentration of the mobile phase, bound compounds are eluted. IEC is widely used in biopharma for purifying proteins and oligonucleotides.
Affinity Chromatography
This method exploits specific interactions between a molecule and a binding partner (e.g., enzyme-substrate or antigen-antibody). Ligands are attached to the stationary phase to capture target molecules from complex mixtures. It’s the method of choice for purifying monoclonal antibodies and enzymes with high specificity.
Supercritical Fluid Chromatography (SFC)
SFC uses supercritical CO₂ as the mobile phase, combining the speed of gas chromatography with the polarity control of liquid chromatography. It’s considered a “green” technique due to reduced solvent usage and is especially useful in chiral separations.
Comparison of Major Chromatography Types
Real-World Analogy & Pharmaceutical Applications
Chromatography may sound technical, but its underlying principle can be seen in everyday life. To explain it simply, imagine you're making coffee with a paper filter. You add ground coffee beans at the top and pour hot water over them.
As the water passes through the filter, it carries along flavors, oils, and caffeine, leaving behind the solids. The resulting brew is a mixture of extracted compounds, each having interacted with the filter differently based on solubility and size.
This is the essence of chromatography: selective movement and separation. Just as different coffee components dissolve and flow through the paper at different rates, molecules in a chromatographic system separate based on how they interact with the mobile and stationary phases.
Now imagine needing to extract only one particular molecule from that coffee—say, just the caffeine. That’s what chromatography enables in scientific labs: isolating individual substances from complex mixtures with pinpoint precision.
Applications in the Pharmaceutical Industry
Chromatography is essential at every stage of pharmaceutical development.
From discovery to final quality testing, it ensures drugs are effective, pure, and safe for human use.
- Drug Discovery
- Impurity Profiling
- Content Uniformity & Potency Testing
- Stability Studies
- Bioanalysis
- Vaccine Development
- Cleaning Validation
Use Case Highlight: Insulin Purification
Insulin, a life-saving peptide hormone for diabetics, is purified using reversed-phase and ion-exchange chromatography. These methods help isolate the correct molecular form, remove byproducts, and ensure bioactivity is retained. The purified product must meet tight specifications for clinical safety and efficacy.
From large pharmaceutical companies to small research labs, chromatography is a cornerstone of analytical testing. Its unmatched ability to dissect complex mixtures with precision makes it the industry’s most relied-upon separation technique.

Core Parameters in Chromatographic Separation
Behind every clean peak on a chromatogram lies a set of finely tuned parameters.
These factors govern how well the compounds in a mixture separate from each other.
Understanding them is critical for method development, system optimization, and troubleshooting.
Let’s explore the most important ones:
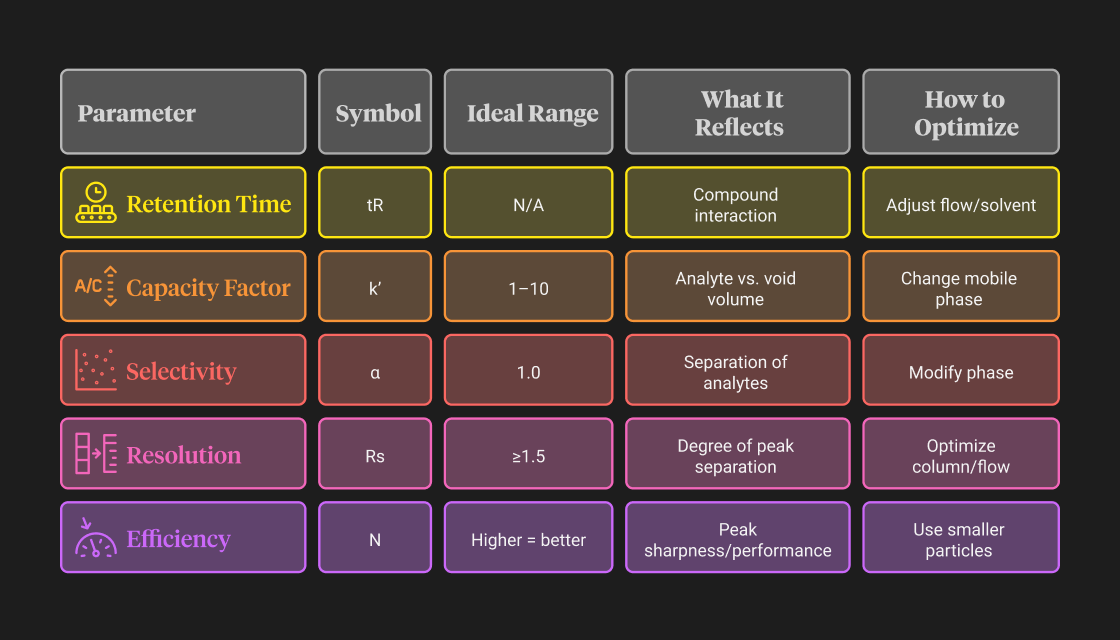
Retention Time (tR)
- Retention time refers to how long a compound takes to travel from the injection point to the detector.
- Each compound has a characteristic tR under a given set of conditions.
- Shorter retention times mean faster elution; longer times may indicate stronger interaction with the stationary phase.
- Consistent retention time is a key sign of system stability.
Capacity Factor (k′)
- The capacity factor expresses how long an analyte is retained relative to the void volume (unretained solvent front).
- An ideal k′ value ranges between 1 and 10 for good resolution and reasonable runtime.
- If k′ is too low, peaks may overlap; if too high, the analysis may be unnecessarily long.
- Adjusting mobile phase composition is a common way to optimize k′.
Selectivity (α)
- Selectivity measures how well two compounds are differentiated by the column.
- It’s the ratio of their capacity factors (k′ values).
- An α value >1.0 indicates the system can distinguish between compounds.
- Improving selectivity often involves changing the stationary phase or modifying the mobile phase pH or polarity.
Resolution (Rs)
- Resolution quantifies how completely two peaks are separated on a chromatogram.
- An Rs value of 1.5 or higher is considered baseline separation.
- Rs depends on three components: efficiency (N), selectivity (α), and capacity factor (k′).
- It can be improved by tweaking column length, particle size, or gradient conditions.
Efficiency (N – Number of Theoretical Plates)
- Efficiency describes how narrow and well-defined a peak is.
- A higher number of theoretical plates (N) means better peak sharpness and improved separation.
- Column efficiency is influenced by particle size, flow rate, temperature, and column packing quality.
Key Chromatographic Parameters
While the science of chromatography is grounded in molecular interactions, its execution depends entirely on precise instrumentation. Each component in a chromatographic system serves a specific function, from moving fluids to capturing signals from separated compounds. Let’s explore the essential instruments that bring chromatography to life in a lab.
Basic Instrumentation Components
Mobile Phase Reservoirs
These contain the solvents used to carry the analyte mixture through the system. They are usually made of glass or inert plastic and are fitted with degassers to eliminate air bubbles that could disrupt flow.
Pumps
The pump generates consistent pressure to push the mobile phase through the column. In HPLC, this pressure ranges from 1000 to 6000 psi; in UHPLC, it can exceed 15,000 psi. Modern systems use dual-piston or quaternary pumps to allow for complex solvent gradients.
Injector/Autosampler
This is where the sample is introduced into the mobile phase stream. Manual injection is possible, but autosamplers improve precision and are essential for high-throughput workflows.
Column
The heart of the system, the column houses the stationary phase and is where the separation actually occurs. Columns vary in material, length, diameter, and particle size depending on the technique and target analytes.
Detector
Detectors sense the presence of compounds as they elute from the column. Different detectors are used based on compound properties (UV-absorption, mass, fluorescence, etc.).
Data System
The data acquisition software collects, processes, and displays the chromatograms. It calculates retention times, peak areas, concentrations, and applies statistical analysis to validate results.
Advanced Instrumentation Features
- Column Ovens: Maintain consistent temperature, improving reproducibility and selectivity.
- Inline Degassers: Prevent baseline noise caused by gas bubbles.
- Multi-detector arrays: Allow simultaneous UV, fluorescence, and MS detection.
- Tandem Configurations: LC–MS/MS, GC–FID–MS, or 2D-LC systems enhance resolution and data richness.
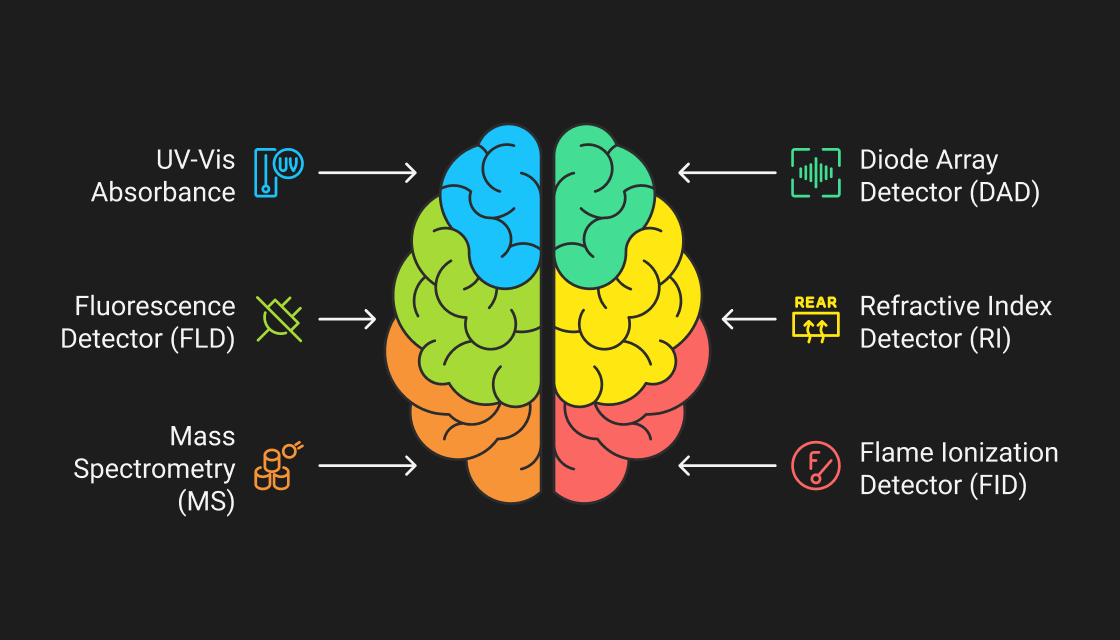
Detection Techniques
Detection is critical—it turns chemical separation into actionable data. Let’s look at some commonly used detectors in chromatography:
Common Chromatographic Detectors
Modern chromatography instruments are built for speed, sensitivity, and automation. They not only separate compounds but also identify, quantify, and validate them with extraordinary precision. This level of control makes chromatography an irreplaceable tool in regulated pharmaceutical environments.
Advantages and Limitations of Chromatography
Chromatography is one of the most versatile tools in modern science. Its strengths span across industries, from pharma and biotech to food safety and forensics. However, like any technology, it has limitations that users must consider during method development and analysis.
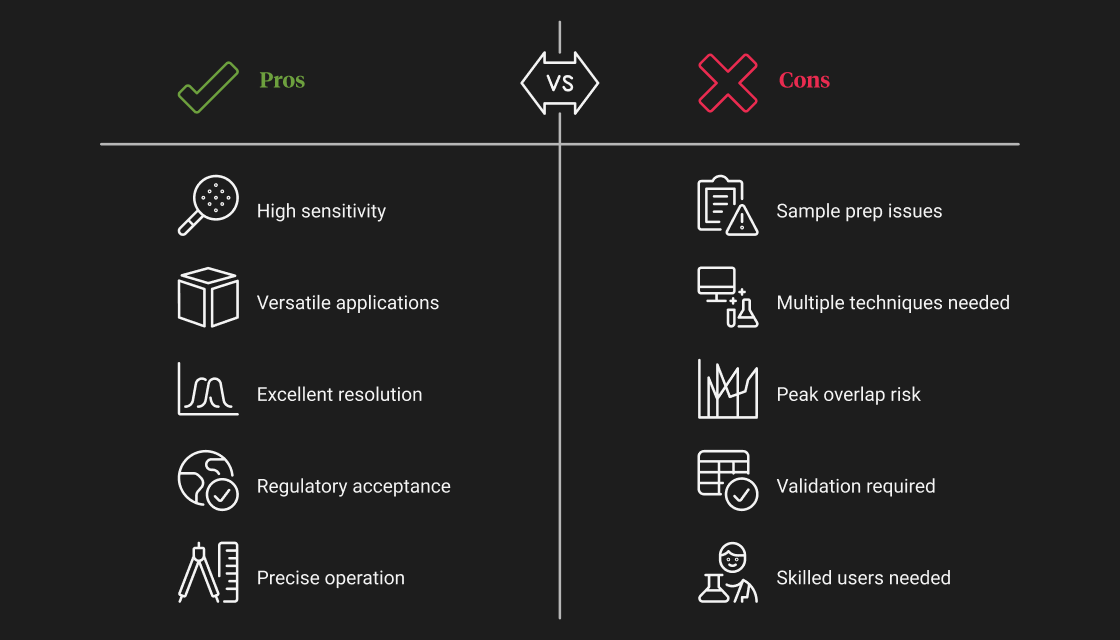
Case Studies & Real-World Applications
Chromatography may seem abstract when discussed theoretically, but its real-world impact is undeniable.
From vaccine testing to forensic analysis, here are some compelling examples of chromatography at work.
Pfizer – UHPLC in mRNA Vaccine Quality Control
When Pfizer developed its COVID-19 mRNA vaccine, speed and quality control were paramount. They used Ultra-High Performance Liquid Chromatography (UHPLC) to confirm RNA integrity, analyze lipid nanoparticles, and detect process-related impurities.
SEC (Size-Exclusion Chromatography) ensured capsid uniformity, while ion-exchange and reversed-phase LC verified purity at every step. Thanks to these chromatographic methods, each vaccine batch underwent over 40 tests before regulatory release.
Roche – LC–MS for Antibody Characterization
Roche, a global biopharma leader, uses liquid chromatography–mass spectrometry (LC–MS) to profile monoclonal antibodies like Rituxan. They assess post-translational modifications, charge variants, and glycan profiles—all critical quality attributes. The integration of LC–MS has allowed faster release timelines, better comparability for biosimilars, and robust in-process monitoring.
WHO – TLC Kits to Detect Counterfeit Medicines
In regions with limited lab access, the World Health Organization (WHO) deploys Thin-Layer Chromatography (TLC) field kits. These simple kits help health workers check for the presence of active pharmaceutical ingredients (APIs) in antimalarials and antibiotics. In Africa and Southeast Asia, TLC helped identify falsified artesunate tablets, leading to lifesaving recalls.
Thermo Fisher – Ion Chromatography for API Purity
Thermo Fisher’s IC systems are used in API (Active Pharmaceutical Ingredient) production to detect residual salts and counterions. Pharma companies rely on ion chromatography to maintain purity and comply with ICH and USP guidelines for inorganic impurities.
Conclusion
That morning, when Dr. Meera watched the crimson hues of her hibiscus tea unfurl, she unknowingly mirrored the foundation of her own work. Just as those natural pigments moved at different speeds through water, she spent her days separating complex drug mixtures using chromatography—searching for clarity in chaos.
Later that day, one anomaly in a chromatographic peak signaled a formulation flaw in a new drug batch. Thanks to that insight, her team avoided costly delays and potential patient risk. It was a quiet reminder: even the smallest deviation on a chromatogram can have life-saving implications.
From that tea cup to the most advanced pharmaceutical lab, chromatography helps scientists read between the lines—uncovering what cannot be seen with the naked eye. It doesn’t just separate molecules; it safeguards medicine, innovation, and lives.
FAQs
1. What is chromatography used for in pharmaceuticals?
Chromatography is used for drug purity testing, impurity profiling, stability studies, content uniformity, and bioanalysis in clinical trials.
2. What are the most common types of chromatography?
HPLC, TLC, Gas Chromatography (GC), Ion Exchange Chromatography, and Size-Exclusion Chromatography are among the most commonly used techniques.
3. Why is chromatography important in quality control?
It ensures that pharmaceutical products meet regulatory requirements for safety, efficacy, and consistency by detecting impurities and confirming identity.
4. What is the role of chromatography in vaccine development?
Techniques like SEC, reversed-phase LC, and ion-exchange chromatography are used to confirm vaccine purity, stability, and structural integrity.




