GSK’s Nucala Approved In China For Chronic Rhinosinusitis With Nasal Polyps Treatment
Nucala gains approval in China as a therapy for chronic rhinosinusitis with nasal polyps.
Breaking News
Jan 04, 2025
Simantini Singh Deo
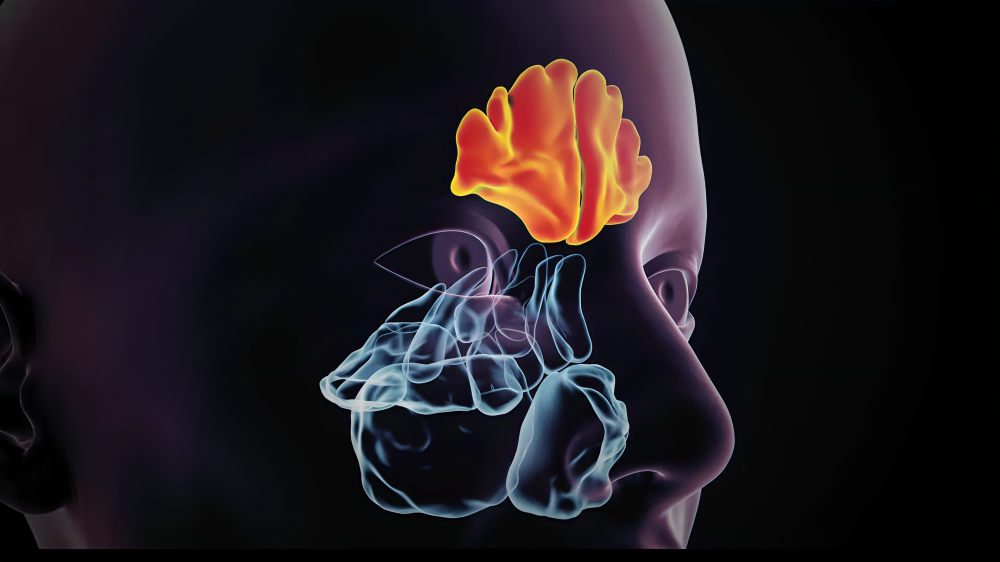
GSK plc announced that the China National Medical Products Administration has approved Nucala (mepolizumab) as a simultaneous therapy with intranasal corticosteroids for adult patients with chronic rhinosinusitis with nasal polyps (CRSwNP). This approval is specifically for individuals whose symptoms remain uncontrolled with systemic corticosteroids and surgery. In China, approximately 107 million people suffer from chronic sinusitis, with about one-third of these cases involving nasal polyps.
"We are delighted that Nucala has been approved in China as a treatment for CRSwNP, a chronic condition for which new and effective treatments are needed. Patients now have a non-surgical option available to them and an alternative to repeated exposure to oral corticosteroids," said Kaivan Khavandi, SVP, Global Head of Respiratory/Immunology R&D at GSK
CRSwNP is characterised by chronic inflammation of the nasal lining, leading to soft tissue growths (nasal polyps) in the nasal cavity and sinuses. This condition consists of symptoms like nasal obstruction, loss of smell, facial pressure, nasal discharge, and sleep disturbances, significantly affecting patients' quality of life. Studies show that up to 80% of CRSwNP cases are driven by type 2 inflammation; blood eosinophil count detects this and is a biomarker measured by simple blood tests. Type 2 inflammation is linked to more severe symptoms and a higher chance of polyp recurrence, even after surgical removal.
The approval of Nucala is based on findings from the phase III MERIT trial, which evaluated the efficacy and safety of mepolizumab over 52 weeks in patients from China, Japan, and Russia with inadequately controlled CRSwNP. These results were further supported by data from the global phase III SYNAPSE trial involving more than 400 patients.
Nucala is already approved in China as an add-on maintenance treatment for severe eosinophilic asthma in individuals aged 12 and older and for adults with eosinophilic granulomatosis with polyangiitis. With this latest approval, mepolizumab offers a promising treatment option for CRSwNP patients struggling to manage their symptoms.
