Nasodine Nears European Approval as Firebrick Pharma Files Marketing Authorisation Application
Firebrick Pharma submits Nasodine nasal spray for European approval, decision expected by December.
Breaking News
Sep 09, 2024
Mrudula Kulkarni
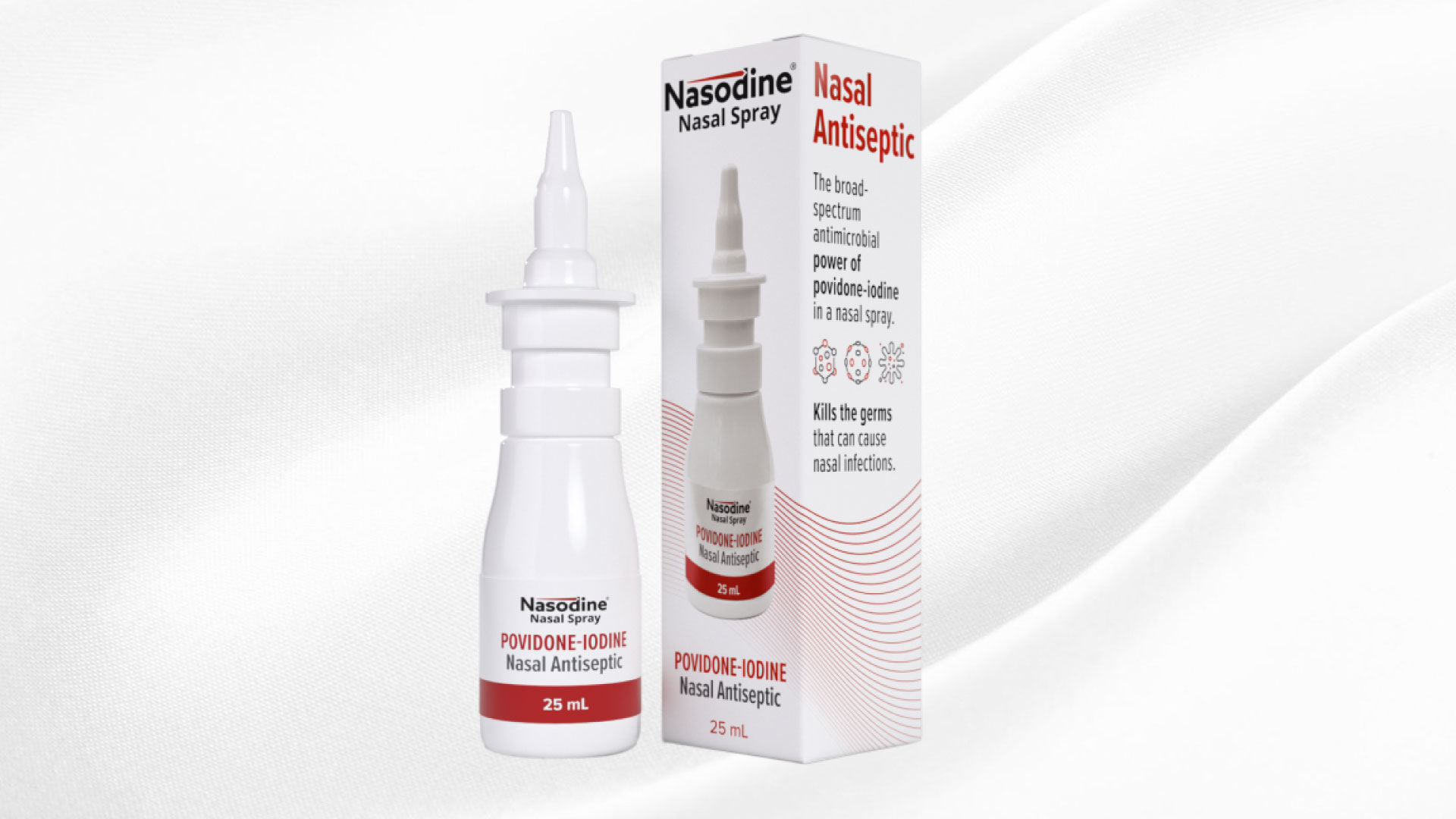
Firebrick Pharma Limited has announced the successful filing
and validation of its Marketing Authorisation Application for Nasodine, a nasal
antiseptic spray, in Europe. This application is currently under review by
European health authorities, with an initial decision expected by 19 December
2024. If approved, Nasodine could be one of the first nasal sprays of its kind
to enter the European market, aimed at providing a new solution for nasal
antisepsis.
Firebrick Pharma is optimistic about the potential impact of
Nasodine and has already laid the groundwork for partnerships within Europe.
The company plans to collaborate with local manufacturing, distribution, and
marketing entities to ensure the product reaches the market efficiently and
effectively. The approval of Nasodine would mark a major milestone for
Firebrick Pharma, significantly expanding its global presence and reinforcing
its position as an innovator in healthcare solutions.
Nasodine’s entry into the European market comes at a time
when nasal antiseptics are gaining attention for their potential to reduce the
spread of infections. Firebrick Pharma’s leadership sees this as a strategic
step toward providing better preventative care, and they remain committed to
ensuring Nasodine’s success in Europe and beyond.
