Galderma's New Skin Treatment Gets FDA Nod, A Victory for Dermatology
FDA clears Galderma's Nemluvio for prurigo nodularis, offering a new option for this underdiagnosed condition.
Breaking News
Aug 14, 2024
Simantini Singh Deo
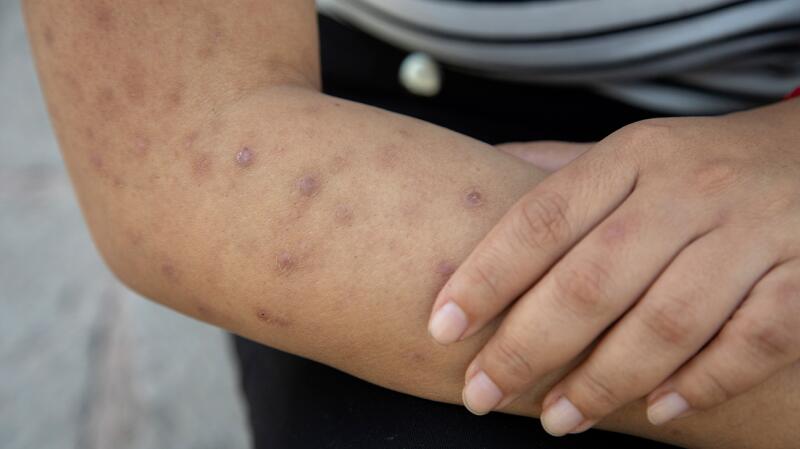
Nemluvio received clearance on Tuesday, nearly two years
after the FDA approved the first treatment for prurigo nodularis—Sanofi and
Regeneron Pharmaceuticals' blockbuster drug Dupixent. Though the two
medications operate through different mechanisms, both aim to address the
inflammatory causes of this underdiagnosed condition, which not only causes
intense itching but also results in the formation of thick, hardened nodules on
the skin. Galderma reports that prurigo nodularis may affect up to 180,000 individuals
in the U.S.
Galderma acquired the rights to Nemluvio, or nemolizumab,
from Chugai Pharmaceutical in 2016. The company progressed the drug to
late-stage clinical trials, testing it on more than 500 adults with prurigo
nodularis to determine if the treatment could reduce itch severity by at least
four points on a scale after four months. In two Phase 3 trials, 56% and 49% of
participants treated with Nemluvio achieved this reduction, compared to just
16% in the placebo groups. The company has submitted a request to the FDA for
approval of Nemluvio for the treatment of moderate-to-severe atopic dermatitis
and anticipates a decision before the end of the year.
Galderma CEO Flemming Ørnskov, who previously led Shire
before its acquisition by Takeda Pharmaceuticals, said “The U.S. FDA’s rapid
approval of Nemluvio in prurigo nodularis is a first step in achieving its
blockbuster platform potential and reinforces our leadership in therapeutic
dermatology.”
