FDA Greenlights SofdraTM for Hyperhidrosis Treatment, Botanix Secures $70M
FDA approves Botanix's SofdraTM for axillary hyperhidrosis; $70M raised, launch in Q3 2024.
Breaking News
Jul 31, 2024
Mrudula Kulkarni
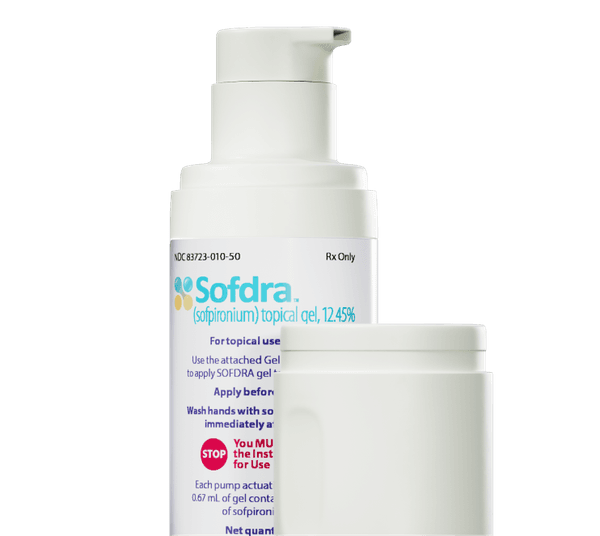
For the purpose of treating primary axillary hyperhidrosis, Botanix Pharmaceuticals Limited's innovative dermatological treatment, SofdraTM, has been approved by the FDA. Through a successful placement, the business has secured $70 million to finance the launch, which is scheduled to start with a patient experience program in Q3 2024 and move into a more comprehensive digital release in Q4. With no debt and a healthy financial position, Botanix is well-positioned to take on the roughly 10 million Americans who suffer from this illness.
