SurGenTec's FDA-Approved B-MAN Bone Marrow Aspirate Kit Revolutionizes Cell Extraction
SurGenTec's B-MAN Kit simplifies bone marrow aspiration, offering high-quality, contaminant-free cells.
Breaking News
Aug 26, 2024
Mrudula Kulkarni
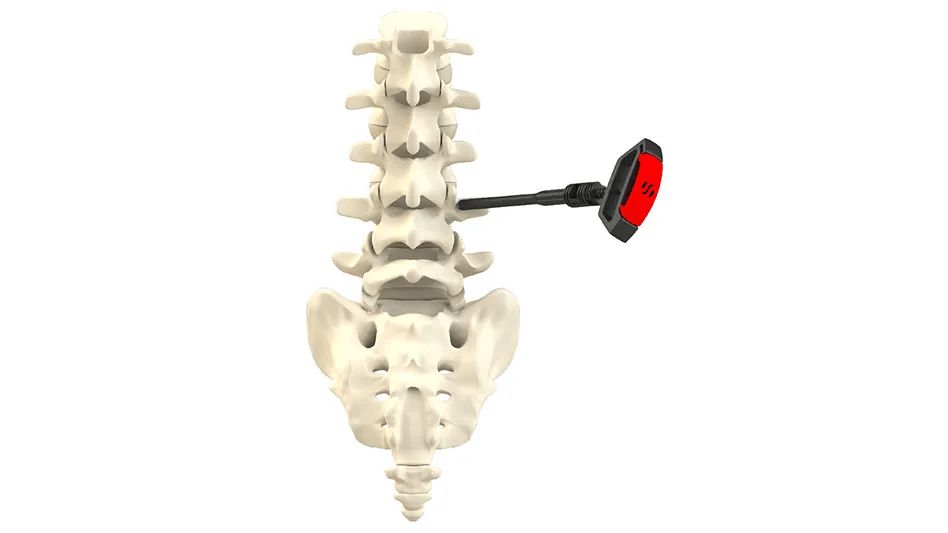
The B-MAN Bone Marrow Aspirate Kit from SurGenTec, which uses an innovative technique to extract high-quality bone marrow with few impurities, has been approved by the FDA. The kit reduces bone spicules and other contaminants using a centrifuge-free technique and CELLect filtering technology, producing a refined aspirate with improved cell viability. As it optimises cell quality, maintains native cell structure, and makes it easier to extract healthier cells, the B-MAN Kit makes aspiration simpler.
Conventional methods of bone marrow aspiration frequently
need centrifuges and several processes, which might be harmful to the integrity
of the cells. The B-MAN Kit facilitates the process of obtaining bone marrow by
making it simple to use and effective. The kit reduces bone spicule and
peripheral blood contamination while increasing the extraction of progenitor
cells by optimising marrow contact with a surface area up to three times larger
than traditional approaches.
In many surgical applications, such as orthopaedic
operations, spinal treatments, and regenerative medicine, bone marrow aspirate
is essential. When a patient's own cells are used instead of those from donors
or allografts, the danger of disease transmission is greatly reduced. .. Based
in Boca Raton, Florida, SurGenTec is a privately held medical device firm that
specialises in cutting-edge technology for orthopaedic and neurosurgical spine
treatments. It places a high priority on patient safety by offering minimally
invasive treatment choices.
