Pelthos Therapeutics Launches ZELSUVMI™, Making Molluscum Contagiosum The First Skin Condition With An FDA-Approved At-Home Treatment
ZELSUVMI: The first at-home FDA-approved gel for molluscum, now available for adults and kids aged 1+.
Breaking News
Jul 11, 2025
Simantini Singh Deo
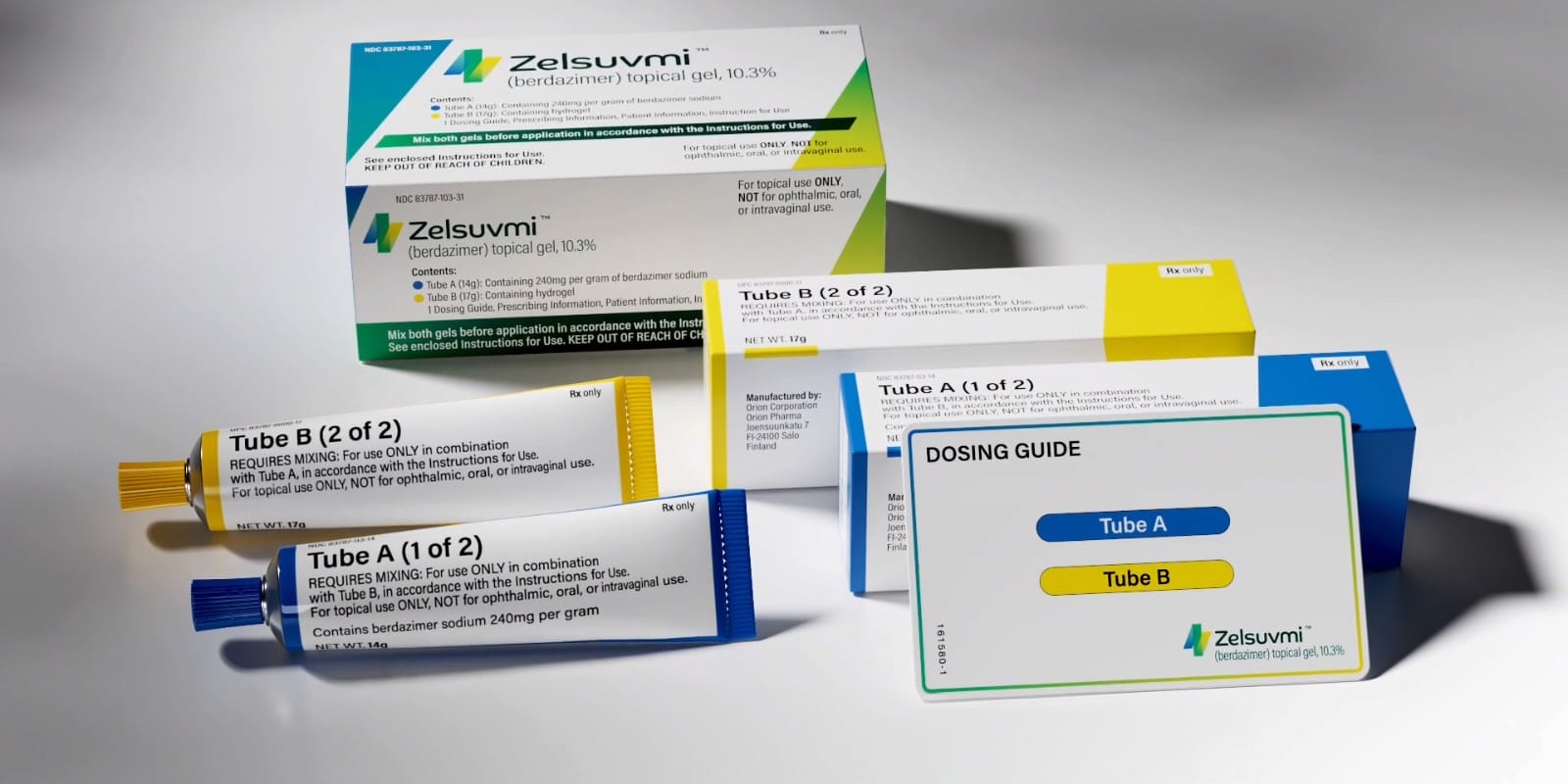
Pelthos Therapeutics Inc., a biopharmaceutical company focused on developing innovative treatments for serious unmet medical needs, has announced the commercial launch of ZELSUVMI™ (berdazimer) topical gel, 10.3%. This new therapy is designed for the treatment of molluscum contagiosum, a common and highly contagious viral skin infection, in both adults and children aged one year and older.
ZELSUVMI received Novel Drug designation from the U.S. Food and Drug Administration (FDA) in January 2024. It is the first and only FDA-approved prescription treatment for molluscum that can be applied at home by patients, parents, or caregivers. This approval provides a much-needed at-home solution for a skin condition that affects millions, especially children, and for which there were previously limited treatment options.
ZELSUVMI is a nitric oxide-releasing topical gel that is applied once daily to affected areas. It is designed for use at the time of diagnosis and offers a combination of convenience, safety, and effectiveness. The gel can be used on various parts of the body, including sensitive areas like the face, groin, and underarms, making it practical for daily use either at home or while on the go.
Scott Plesha, CEO of Pelthos, said in a statement, “We believe that the commercial launch of ZELSUVMI marks a significant advancement for patients with molluscum and their caregivers, who previously lacked an at-home treatment option for this burdensome skin infection. We are excited to make ZELSUVMI widely available for the millions of patients afflicted by this condition. Our commercial efforts will aim to provide stellar education and support for patients seeking effective treatment for their molluscum infections.”
Nanette Silverberg, MD, Chief of Pediatric Dermatology at the Mount Sinai Health System, stated, “Many parents delay seeking treatment for their children’s uncomfortable lesions because current procedural treatments and frequent office visits can be inconvenient, while therapeutic options are limited. Untreated molluscum can spread throughout the child’s body but also to other family members. A safe and effective topical gel for molluscum, like ZELSUVMI, which can be applied at home or on the go, would make a significant difference for this young patient population and address a serious, unmet medical need.”
Sai Rangarao, Chief Commercial Officer at Pelthos, mentioned, “We are launching our ZelsuvmiGo patient support program, which we expect to help onboard patients seamlessly and provide resources for caregivers. To ensure that ZELSUVMI reaches the people who need it quickly, we have hired 50 sales territory managers across the country to work with physicians who treat a high volume of patients with molluscum. We have also implemented extensive digital outreach and awareness efforts to ensure ZELSUVMI can be prescribed by any healthcare provider at any time. These patients have waited a long time for an at-home treatment option.”
The approval of ZELSUVMI was supported by a large Phase 3 clinical trial, which is the largest randomized study conducted to date for the treatment of molluscum. This multicenter, double-blind, vehicle-controlled study included 891 participants. After 12 weeks of treatment, nearly 33% of patients who received ZELSUVMI experienced complete clearance of their molluscum lesions, compared to just 19.7% in the placebo group.
Many patients also began to see results within the first two weeks of use. With this launch, Pelthos Therapeutics introduces an important new option for patients and families dealing with molluscum. ZELSUVMI offers a convenient, non-invasive, and effective way to manage and treat this viral skin condition without needing in-office procedures or multiple healthcare visits.
