by Vaibhavi M.
6 minutes
7 Ethical Pharmaceutical Marketing Practices High-Performing Brands Follow
Seven ethical pharmaceutical marketing practices that help high-performing brands build trust, ensure compliance, and drive sustainable growth.
20260119122843.webp)
In the highly regulated pharmaceutical industry, marketing is not just about visibility or brand recall; it is about trust, compliance, and patient safety. Unlike fast-moving consumer sectors, pharmaceutical marketing operates under strict regulatory oversight, ethical scrutiny, and public accountability. High-performing pharmaceutical brands understand that sustainable growth is achieved not through aggressive promotion but through ethically sound, scientifically accurate, and patient-centric marketing practices.
As regulatory bodies such as the FDA, EMA, CDSCO, and WHO, along with industry codes such as IFPMA and UCPMP, continue to tighten oversight, ethical marketing has evolved from a “best practice” to a business necessity. Brands that consistently outperform their peers do so by embedding ethics into every aspect of their commercial strategy.
This article explores seven ethical pharmaceutical marketing practices that high-performing brands follow, and why these principles are essential for long-term success.
1. Prioritising Scientific Accuracy Over Promotional Hype
At the core of ethical pharmaceutical marketing lies scientific integrity. High-performing brands ensure that every marketing claim is firmly supported by peer-reviewed clinical data, approved product labels, and regulatory-cleared materials.
Ethical brands avoid exaggeration, selective data presentation, or over-interpretation of trial outcomes. For example, efficacy claims are always contextualised with study design, patient population, endpoints, and limitations. Safety information is presented with equal prominence, including known adverse effects, contraindications, and risk mitigation strategies.
This approach aligns with regulations such as:
- FDA’s Prescription Drug Advertising Rule
- EMA’s Directive 2001/83/EC
- India’s UCPMP guidelines
By respecting scientific nuance, ethical marketers build credibility with healthcare professionals (HCPs) and reduce the risk of regulatory action, product recalls, or reputational damage. This principle is central to responsible pharmaceutical content marketing.
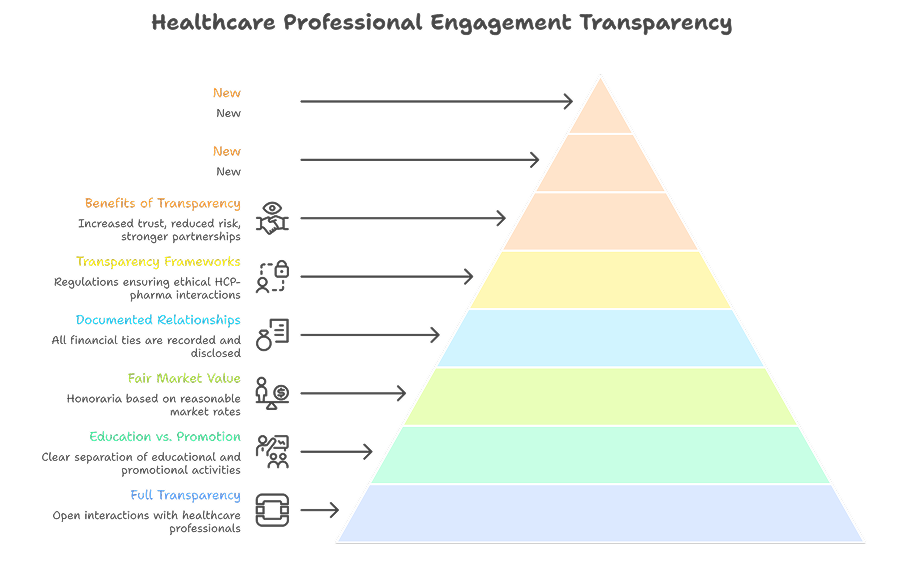
2. Ensuring Transparency in Healthcare Professional Engagement
Ethical pharmaceutical brands maintain full transparency in their interactions with healthcare professionals, whether through educational programs, advisory boards, or conference sponsorships.
Rather than using incentives to influence prescribing behaviour, high-performing companies clearly separate education from promotion. Speaker programs are scientifically driven, honoraria are based on fair-market value, and all financial relationships are documented and disclosed where required.
Transparency frameworks such as:
- The Sunshine Act (US)
- EFPIA Disclosure Code (Europe)
- Voluntary disclosure initiatives in Asia and India
help ensure that relationships between pharma companies and HCPs remain professional, ethical, and patient-focused. Brands that embrace transparency often enjoy greater trust, reduced compliance risk, and stronger professional partnerships.
3. Adopting a Patient-Centric Marketing Approach
Ethical pharmaceutical marketing extends beyond physicians to include patients and caregivers, but always within regulatory boundaries. High-performing brands design patient-facing communications that are educational, balanced, and empowering, rather than fear-based or misleading.
This includes disease awareness campaigns that:
- Focus on early diagnosis and treatment options
- Avoid direct product promotion where prohibited
- Use simple, medically accurate language
Patient support programs, adherence tools, and digital health platforms are positioned as value-adding services, not disguised promotional tactics. Importantly, patient data collected through these initiatives is handled in strict compliance with data privacy laws, including GDPR, HIPAA, and India’s DPDP Act.
By respecting patient autonomy and privacy, ethical brands strengthen long-term loyalty and public trust. This reflects a broader shift toward patient-centric pharma marketing models.
4. Maintaining Ethical Digital and Omnichannel Marketing Practices
As pharmaceutical marketing increasingly shifts to digital platforms, ethical responsibility has expanded to include online content, social media, AI-driven targeting, and communication of real-world evidence.
High-performing pharma brands apply the same ethical rigor online as they do offline. Digital campaigns undergo medical, legal, and regulatory (MLR) review, ensuring that content remains compliant across geographies.
Ethical digital practices include:
- Clear separation between promotional and educational content
- Accurate handling of off-label discussion restrictions
- Responsible use of cookies, tracking, and AI analytics
Rather than exploiting digital tools for aggressive targeting, ethical marketers use them to deliver relevant, scientifically sound information to the right audience, enhancing engagement without compromising compliance. Executing ethical campaigns at scale requires disciplined omnichannel pharma marketing.
1(1)20260119114830.png)
5. Aligning Marketing with Regulatory and Compliance Frameworks
Ethical pharmaceutical marketing is built on proactive compliance, not reactive correction. High-performing brands integrate regulatory considerations early in campaign planning, rather than treating compliance as a final checkpoint.
This includes close collaboration between:
- Marketing teams
- Medical affairs
- Regulatory affairs
- Legal and compliance departments
By embedding compliance into strategy, companies reduce approval delays, prevent costly rework, and ensure consistency across global markets. Ethical brands also invest in regular compliance training for sales and marketing teams, keeping them updated on evolving regulations and ethical standards.
Compliance, when done right, becomes a competitive advantage rather than a constraint. This approach is reinforced by proactive pharma regulatory compliance strategies.
6. Avoiding Inducements and Unethical Sales Incentives
One of the clearest markers of ethical pharmaceutical marketing is the elimination of inducements that could influence prescribing decisions. High-performing brands move away from volume-based incentives and instead focus on value-based engagement.
Sales teams are trained to act as scientific partners, not transactional promoters. Their performance metrics increasingly include:
- Quality of scientific discussions
- Compliance adherence
- Relationship strength with HCPs
By shifting from aggressive sales targets to ethical engagement models, companies reduce regulatory risk and foster long-term professional respect. Such models are increasingly replacing traditional push vs pull pharma marketing approaches.
7. Measuring Success Beyond Sales Numbers
120260119115703.png)
Ethical pharmaceutical brands redefine success. While revenue growth remains important, it is not the sole metric. High-performing companies evaluate marketing effectiveness through a broader lens that includes:
- Quality of HCP engagement
- Knowledge transfer effectiveness
- Patient outcomes and adherence improvements
- Compliance audit results
- Brand trust and reputation metrics
This long-term perspective recognizes that ethical marketing builds sustainable brands, resilient to regulatory changes and public scrutiny. Many organizations now track impact using data-driven pharma marketing metrics. Companies that prioritise ethics consistently outperform peers during market disruptions, policy changes, and public health crises.
Why Ethical Pharmaceutical Marketing Drives Long-Term Performance
Ethical marketing is not just about avoiding penalties; it is about building enduring value. Brands that follow ethical pharmaceutical marketing practices benefit from stronger stakeholder trust, smoother regulatory interactions, and enhanced brand equity.
In an industry where credibility directly impacts patient outcomes, ethical behaviour is inseparable from commercial success. As healthcare systems demand greater accountability, high-performing pharmaceutical brands will continue to lead by aligning science, ethics, and strategy.
Ethical pharmaceutical marketing is no longer optional—it is the foundation of modern, responsible pharma leadership.
FAQs
1. What is ethical pharmaceutical marketing?
Ethical pharmaceutical marketing promotes medicines responsibly using scientifically accurate, compliant, and patient-focused communication.
2. Why is ethical marketing important in the pharma industry?
It ensures patient safety, regulatory compliance, and long-term trust among healthcare professionals and the public.
3. How do pharma companies ensure marketing compliance?
Through medical-legal review processes, regulatory alignment, and continuous compliance training.
4. What role does patient-centricity play in pharma marketing?
It focuses on education, adherence, and support while respecting patient privacy and autonomy.
5. Can ethical pharma marketing improve business performance?
Yes, it builds credibility, reduces regulatory risk, and supports sustainable growth.






