by Ravindra Warang
7 minutes
3D Bioprinting in Regenerative Medicine: Hype vs Reality
Explore the real-world progress, promises, and limits of 3D bioprinting — from printed skin to the future of transplantable organs.
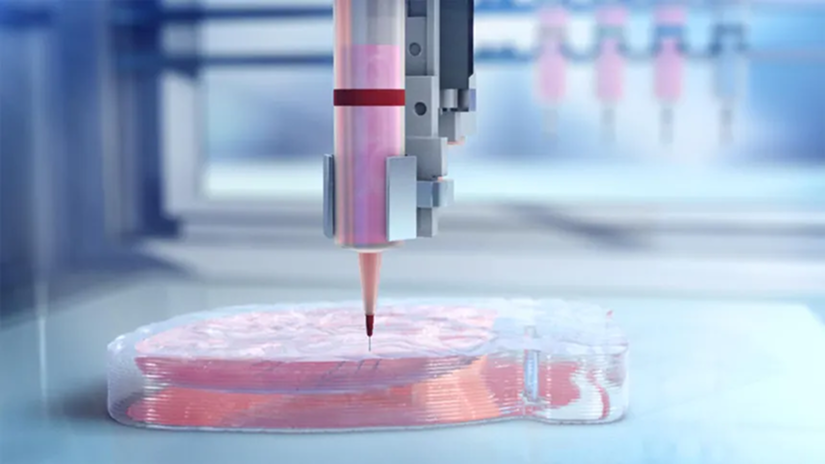
In 2019, a team of scientists in Tel Aviv made headlines by bioprinting a tiny, beating human heart. This groundbreaking achievement in 3D bioprinting regenerative medicine wasn’t ready for transplant, but it featured blood vessels and chambers made from the patient’s own cells. To the world, it looked like science fiction leaping into reality.
In the years since, 3D bioprinting has captured the imagination of regenerative medicine. From cartilage scaffolds to skin grafts and kidney patches, the idea of printing human tissue on demand is no longer confined to labs — it’s on the roadmap of hospitals, biotech firms, and even space agencies. These biotechnology advancements are paving the way for patient-specific therapy that could revolutionize how we approach healing and recovery.
But how much of this is real? How much is hype? And what are we actually printing today versus what’s still a distant dream? As we explore the organ printing potential of 3D bioprinting, it’s essential to consider factors like cell viability in bioprinting and the importance of biochemical microenvironment mimicry.
Let’s separate fact from fantasy — and explore where 3D bioprinting truly stands in regenerative medicine. We’ll also discuss regulatory considerations such as FDA guidelines for 3D printed medical products to ensure a comprehensive understanding of this rapidly evolving field.
What Is 3D Bioprinting?
3D bioprinting is a process that uses specialized printers to deposit layers of bioink — a mixture of cells, biomaterials, and growth factors — to build living structures. Just like traditional 3D printing, it works layer-by-layer. But instead of plastic or metal, it uses living components.
These printed constructs can range from:
- Simple acellular scaffolds for wound healing
- Cell-laden constructs for cartilage or liver tissue
- Vascularized organ models for drug testing
- Experimental organ patches or prototypes for transplantation
Core Components of 3D Bioprinting
The core components of 3D bioprinting include:
- Bioink: A combination of hydrogels and cells (such as stem cells or chondrocytes)
- Printers: Various printing systems including extrusion-based printing, inkjet bioprinting, and laser-assisted bioprinting
- Designs: Computer-aided design (CAD) models representing tissues or anatomical segments
- Bioreactors: Chambers used to mature and condition the printed tissues
The Process of 3D Bioprinting
Bioprinting doesn’t create finished organs overnight — it’s a staged, evolving process with multiple biological checkpoints. The journey often begins with pre-bioprinting modeling and imaging acquisition to ensure precise design and functionality.
As the field advances, companies like Organovo Holdings, ROKIT Healthcare, and Oxford Performance Materials are at the forefront, exploring the hierarchical structural properties of tissue engineering and investigating challenges and future opportunities in scaling up bioprinted tissues/organs.
Why Bioprinting Matters in Regenerative Medicine
Traditional methods of regenerative medicine — such as using donor grafts, scaffolds, or stem cell injections — often have limitations in terms of structure, integration, or function. This is where the advancements in 3D bioprinting technology become crucial.
3D bioprinting offers key advantages:
- Personalization: Custom personalized tissues based on patient scans
- Precision: Microscale cell placement at microscale accuracy
- Scalability: Potential for automated tissue production to streamline complex tissue creation
- Integration: Ability to include vasculature integration or nerves from the start
In areas like orthopedics, dermatology, ophthalmology, and cardiology, this level of precision can greatly enhance outcomes — both in terms of appearance and function.
Hospitals are already experimenting with bioprinted solutions for various conditions:
- Bioprinted cartilage for knee repairs
- Skin grafts made from bioprinted tissue for burn victims
- Corneal layers created through bioprinting for restoring vision
However, it's important to note that simply creating these tissues through bioprinting isn't enough. Additional techniques are required after the printing process to ensure that the tissues mature properly and function as intended.
As companies like Tissue Engineering Inc. continue to push boundaries in this field, new tools are emerging that will transform how we tackle complex regenerative issues. For instance, the Commercial Blood Vessel Bioprinter developed by Digilab is set to change the game when it comes to creating intricate blood vessels.
Moreover, certain factors play a critical role in achieving successful bioprinting results:
- Bioink viscosity optimization: Finding the right thickness or consistency for the bioink used in printing
- Bioink gelation and stabilization: Ensuring that the printed tissues maintain their shape and structure over time
These advancements will not only improve existing applications but also open doors for innovative solutions in regenerative medicine.
To learn more about how imaging technologies work hand-in-hand with computer-aided design (CAD) for accurate tissue modeling and further enhance these cutting-edge strategies, click here.
Where We Are Today — What’s Real and Available
Despite dramatic headlines, most of today’s applications in 3D bioprinting regenerative medicine are still preclinical or early-stage clinical. Here’s what’s truly happening:
- Bioprinted skin grafts: Used experimentally in burn units and for diabetic wound healing. These bioprinted skin grafts are often acellular or contain fibroblasts and keratinocytes.
- Cartilage patches for osteoarthritis: In development for knee and joint injuries, these cartilage patches help avoid full joint replacement.
- Bone scaffolds: Printed with bioceramics or stem cell-loaded bioinks, these bone scaffolds are used in maxillofacial reconstruction and bone defect healing.
- Corneal implants: Semi-transparent, curved layers of collagen and cells are being tested for corneal repair in regions with donor shortages.
- Liver and kidney tissues for toxicity screening: Not full organs, but small patches used for toxicity screening and disease modeling in the lab.
So while full-sized, transplantable organs like hearts or kidneys are still years away, functional tissue segments are already in international pilot programs across Europe, Japan, and the US. As advancements continue in bioink design concepts and biocompatibility in bioinks, the potential for 3D printed organs becomes increasingly viable. Additionally, innovations in rapid prototyping for tissue scaffolds fabrication and techniques such as bioprinter nozzle precision control are essential for ensuring success in these endeavors.
Case Studies – Bioprinting in Action
Let’s explore a few high-impact use cases proving the real-world potential of bioprinting applications:
1. Wake Forest Institute for Regenerative Medicine (USA)
Their team has bioprinted human skin and urethral structures used in reconstructive surgeries. They’re also developing tissue patches for battlefield injuries in partnership with the U.S. military, showcasing the innovative use of medical imaging technology and engineering living organs with 3D bioprinting techniques.
2. CELLINK/BICO (Sweden)
Using the CELLINK/BICO Bio X printer, which is now widely adopted in academic labs and biotech firms, researchers are printing tissues like cartilage, liver, and heart valves. This advanced bioprinting technology has been instrumental in personalized drug testing and creating patient-specific 3D models for printing that aid in tissue modeling.
3. Poietis (France)
Pioneering laser-assisted bioprinting for hair follicle regeneration and advanced skin constructs, Poietis utilizes dynamic printing methods that allow real-time cell placement monitoring. Their work exemplifies the cutting edge of Micro/nanofabrication techniques in the bioprinting field.
4. T&R Biofab (South Korea)
T&R Biofab has gained FDA approval for 3D-printed ear cartilage implants designed specifically for microtia patients—one of the first personalized regenerative implants cleared for use. This milestone highlights the integration of computer-aided manufacturing (CAM) processes in developing complex biological structures.
These breakthroughs aren’t marketing stunts—they’re blueprints for what’s possible when bioprinting is scaled and clinically aligned, demonstrating the vast potential of bioprinting applications across various medical fields.
What’s Still Hype — And Why We’re Not Printing Whole Organs Yet
Despite optimism, several claims about bioprinted organs remain hopeful:
- Bioprinted hearts or lungs for transplant are not available and remain extremely complex due to the need for full vascular networks in organs, muscle coordination, and immune compatibility.
- Multi-organ systems that function as living machines inside the body are in prototype phases at best — often limited to organ-on-chip studies.
- Consumer-ready bioprinting challenges such as biological maturity, quality control, and regulatory approval for bioprinted organs hinder the development of “print-your-own-tissue” ideas.
Why the delay?
- Building an organ isn’t just architecture — it’s biology. Cells need to communicate, receive blood, oxygen, and mechanical cues.
- Vascularization remains a major roadblock. Without blood flow, most thick tissues die beyond a few hundred microns.
- Regulatory frameworks for implantable, living, and patient-specific tissues are still evolving.
So yes — bioprinting is exciting. But printing a fully functional, transplantable organ involves many unsolved problems in materials science in bioprinting, stem cell biology, and systems integration. Challenges such as ensuring cell viability in bioprinting processes and effective vascular and neural integration in organ printing are crucial. Additionally, advancements in technologies like computed tomography (CT) and magnetic resonance imaging (MRI) play vital roles in scaffold fabrication in tissue engineering and assessing the complexity of multi-organ systems. As we navigate these hurdles, staying informed about FDA guidance on 3D printed medical devices will be essential for progress in this innovative field.
What’s Coming Next — Near-Term Applications in 3D Bioprinting Regenerative Medicine
Here’s what we can realistically expect in the next 3–5 years in bioprinting:
- Customized skin for burns and wound care, especially in military and trauma settings. This advancement will enhance healing processes by accurately replicating complex tissue architecture.
- Cartilage bioprints for sports injuries, offering alternatives to invasive surgeries and promoting faster recovery through regenerative bioprinting techniques.
- Vascular grafts and stents for bypass surgeries or dialysis access, improving patient outcomes with tailored solutions that address specific physiological needs.
- Bioprinted corneal implants for vision restoration, particularly in regions with low donor availability, ensuring that patients receive effective treatment options.
- Liver tissue chips for drug screening, especially for rare or pediatric diseases, facilitating in vitro tissue models creation that better mimic human responses.
These innovations are not dreams — they are either in clinical trials in bioprinting or receiving regulatory attention. As we continue to understand fundamental principles of 3D bioprinting in regenerative medicine, companies like EnvisionTEC GmbH and Advanced Solutions Life Sciences are at the forefront of these developments, utilizing techniques such as inkjet 3D bioprinting and stereolithography to revolutionize healthcare.
Key Challenges Still Holding Back the Field
Even with immense promise, bioprinting must overcome several barriers:
- Bioink limitations
Most current bioinks are hydrogels with low mechanical strength. Mimicking native tissue stiffness, conductivity, or elasticity is a challenge. These bioink limitations hinder the development of functional tissues in additive manufacturing in regenerative medicine.
- Vascularization challenges
Without blood vessels, printed tissues can’t survive or integrate beyond a few millimeters in thickness. Addressing vascularization challenges is crucial for creating viable structures that support organ transplantation shortage solutions.
- Cell sourcing issues
Getting enough viable, differentiated cells (especially patient-specific) is difficult, particularly for complex organs. These cell sourcing issues complicate the production of tissues that meet the standards of clinical-grade production.
- Regulatory gaps in bioprinting
Bioprinted tissues don’t neatly fit into drug, device, or biologic categories. Regulatory gaps in bioprinting pose significant obstacles as approval pathways are still evolving, especially in light of FDA guidance on additive manufacturing.
- Cost and scalability of bioprinting
Many printers and bioreactors are expensive. Scaling up from lab prototypes to clinical-grade production is resource-intensive. Understanding the cost and scalability of bioprinting is essential for advancing the field and ensuring that technologies like those developed by Organovo and Cyfuse Biomedical K.K. become widely accessible.
As you explore these challenges, consider learning about different 3D bioprinting techniques and their applications in tissue engineering to gain a comprehensive understanding of the 3D bioprinting reality.
The Role of AI and Automation in Bioprinting’s Future
Artificial intelligence (AI in bioprinting) is now being used to:
- Optimize print path and layer fidelity through advanced print path optimization techniques
- Simulate tissue maturation and vascular growth using sophisticated tissue maturation simulation methods
- Standardize quality control for printed constructs, ensuring rigorous quality control in bioprinting processes
- Predict degradation, rejection, or fusion outcomes effectively
Combined with robotics in bioprinting, AI will drive faster, safer, and more reproducible printing — making clinical-grade tissue production more accessible and affordable. This integration of automation in tissue production is revolutionizing the field.
Bioprinting isn’t just a biological challenge — it’s an engineering and data science frontier as well. As we explore the role of bioinks and biomaterials in organ fabrication using bioprinting technology, it’s essential to consider various techniques such as extrusion-based bioprinting and laser-assisted 3D bioprinting (LAB).
Moreover, the development of transplantable bioprinted organs is becoming increasingly viable with the help of preclinical drug screening models that utilize advanced bioprinting methods.
Conclusion: The Promise and the Print
3D bioprinting in regenerative medicine isn’t science fiction anymore — but it’s not quite science routine either. We’re living in the in-between: where patches are real, organs are possible, and the potential is massive.
What’s hype? The idea that we’ll print a heart next year.
What’s reality? We’re already exploring current applications of bioprinting by printing skin, cartilage, and liver tissues for research and pilot therapies.
What’s next? Bridging the biological gaps in organ printing with smarter design in bioprinting, better bioinks, and multidisciplinary collaboration in regenerative medicine.
The road is long, but every printed patch, vessel, or scaffold brings us closer to the future of organ printing — a day when we don’t just repair organs — we print them using advanced 3D printing techniques.
As we advance, discovering how medical imaging integrates with 3D bioprinting for patient-specific organ regeneration will be crucial. Companies like TeVido BioDevices and Hewlett-Packard are at the forefront of this revolution, utilizing methods such as stereolithography-based bioprinting (SLB) to create biocompatible implantable constructs that could redefine medical devices as we know them.
FAQs (Frequently Asked Questions)
Q1: What is 3D bioprinting and how does it work?
3D bioprinting is a cutting-edge technology that uses specialized printers to deposit layers of bioink—composed of living cells, biomaterials, and growth factors—to construct complex living structures. This process involves using computer-aided design (CAD) models to precisely fabricate tissues and organ prototypes, followed by maturation in bioreactors.
Q2: Can we print entire organs today?
Not yet. While we can print small tissue sections and prototypes—often referred to as bioprinted organ availability—full-sized, transplantable organs like hearts or kidneys remain in early research stages due to complexity and vascularization issues. Identifying challenges and future perspectives in 3D organ bioprinting development is essential for understanding the limitations we face.
Q3: How is AI helping the bioprinting field?
AI's role in bioprinting development is transformative; it simulates tissue growth, optimizes printing precision, predicts biological behavior, and improves reproducibility. By accelerating development and reducing trial-and-error processes, AI is revolutionizing how we approach complex tasks within the 3D bioprinting landscape.
Q4: What are the main applications of 3D bioprinting in medicine?
3D bioprinting has diverse medical applications including creating wound healing scaffolds, cartilage and liver tissue constructs, organ models for drug testing, and organ prototypes or patches for transplantation. Clinically, it is used for bioprinted skin grafts for burns and diabetic wounds, cartilage patches for osteoarthritis, bone scaffolds for maxillofacial reconstruction, corneal implants in areas with donor shortages, and liver/kidney tissues for toxicity screening and disease modeling.
Q5: How is artificial intelligence (AI) enhancing the field of 3D bioprinting?
AI plays a vital role in optimizing print paths for precision printing, simulating tissue maturation and vascular growth processes, standardizing quality control measures to ensure reproducibility, and predicting biological outcomes. These AI-driven advancements improve efficiency and accuracy in bioprinting workflows.




