by Ravindra Warang
7 minutes
Environmental Monitoring in Cleanrooms: A Step-by-Step Guide
A step-by-step 2025 guide to cleanroom EM in pharma—from viable sampling to trending, SOPs, and real-world compliance tips.
In 2019, a mid-sized injectable facility in Europe was forced to halt production after multiple batches failed sterility testing. A post-mortem audit revealed the issue: elevated microbial levels in the Grade B zone—flagged by the Environmental Monitoring (EM) team weeks earlier but dismissed as anomalies. This incident underscores the critical role of environmental monitoring cleanroom practices in maintaining sterility assurance.
The cost? Three months of downtime, a damaged reputation, and lost regulatory trust.
This real-world event is a stark reminder: cleanrooms are only as sterile as their surveillance systems. Effective environmental monitoring is not just a compliance checkbox—it's the pulse-check of cleanroom health, essential for GMP compliance and robust contamination risk management. In this article, we’ll break down a step-by-step framework for designing, implementing, and optimizing an EM program in pharmaceutical cleanrooms.
We will explore key aspects such as:
- ISO cleanroom standards
- The importance of monitoring particulate contamination
- How biofilms can impact sterility during aseptic process validation media fill tests
- The classifications and zoning of pharmaceutical cleanrooms and their impact on monitoring strategies
- How to interpret environmental monitoring data trends to detect deviations early and implement corrective actions promptly
What is Environmental Monitoring (EM)?
Environmental monitoring is the systematic collection and analysis of data related to the cleanroom environment. It is crucial for detecting viable and non-viable contamination, including both microbial and particulate contamination, to ensure a safe and sterile environment.
EM Ensures:
- Sterility assurance during aseptic operations
- Compliance with GMP and ISO standards, including regulatory compliance for cleanrooms like GMP Annex 1 FDA ISO14644
- Early detection of contamination risks through effective contamination risk assessment
- Data for trend analysis and corrective action, supporting data trend analysis efforts in cleanroom monitoring protocols
By implementing robust environmental monitoring practices, such as using Particle Monitoring Systems and Surface Sampling (Contact Plates and Swabs), facilities can maintain their Contamination Control Strategy (CCS) effectively.
Understanding the environmental monitoring definition is essential for identifying common sources of microbial contamination within cleanrooms and how to control them effectively. Additionally, adhering to standards like Federal Standard 209E (FS-209E) and ISO 14644 ensures that all EM SOPs are aligned with industry best practices.
Key Elements of a Cleanroom EM Program
1. Viable Monitoring
Viable monitoring techniques involve sampling and culturing living microorganisms using:
- Settle Plates (passive air sampling)
- Contact Plates (surface monitoring)
- Swabs (irregular surfaces)
- Active Air Samplers (volumetric microbial sampling)
These methods are essential for ensuring compliance with FDA Aseptic Guidelines and understanding cleanroom classifications and standards for pharmaceutical environments.
2. Non-Viable Monitoring
Non-viable monitoring methods focus on airborne particles using:
- Laser Particle Counters
- Real-time sensors with continuous monitoring
These tools are crucial for effective airborne particle control and adhering to Good Manufacturing Practice (GMP) standards.
3. Personnel Monitoring
Personnel monitoring in cleanrooms assesses microbial shedding from gown, gloves, and exposed skin areas. This process, known as microbial shedding assessment, is vital for maintaining contamination control and ensuring the integrity of the cleanroom environment.
4. Surface and Equipment Monitoring
Surface and equipment monitoring examines cleanroom-critical surfaces such as:
- Workstations
- Door handles
- LAF benches
- Filler nozzles
This cleanroom-critical surface monitoring ensures that both viable/non-viable particles are effectively managed, reducing the risk of contamination across different cleanroom zones.
Step-by-Step: Setting Up an EM Program
Step 1: Risk Assessment & Zoning
Conduct a thorough risk assessment in cleanrooms using a contamination control strategy (CCS) to:
- Map cleanroom zoning (Grade A to D)
- Identify critical control points
- Define alert/action levels
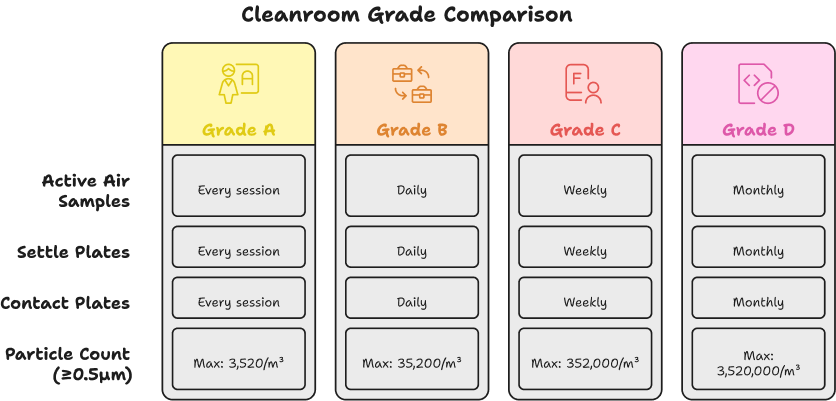
Step 2: Define Sampling Plan
Design a sampling plan definition based on:
- Room classification
- Process type (aseptic vs non-sterile)
- Historical trend data
Consider learning about particle contamination types and sources in cleanrooms to enhance your understanding of the environment.
📌 Example:
Step 3: Choose Equipment and Media
- Use validated active air samplers (≥1 CFU sensitivity) for effective environmental monitoring cleanroom practices.
- Select contact plates with neutralizing agents to ensure accurate microbial detection.
- Ensure media is growth-promoting and complies with USP <61> and <62>
Step 4: Train the EM Team
Personnel must be trained in:
- Aseptic sampling technique
- Gowning discipline
- Proper documentation
Incorporate training on Isolator/RABS Sampling techniques and the importance of HEPA filter effectiveness in pharmaceutical cleanrooms.
Step 5: Establish Data Review Protocol
- Define alert/action levels for each sampling point
- Automate trending using EM software or eQMS
- Escalate excursions promptly—be prepared with an EM excursion response plan.
This data review protocol is essential for maintaining compliance with regulations such as EU Annex 1.
Understanding Trends in Cleanroom EM
Why Trends Matter
An isolated incident of cleanroom environmental monitoring (EM) trending is a cause for concern. However, if you notice three such incidents within a month, it indicates a trend that needs attention.
Tools for Analyzing EM Data
To effectively analyze EM data and identify trends, it's crucial to utilize statistical tools. Here are some recommended tools for EM data analysis:
- Moving averages
- Box plots
- Control charts
These statistical tools can assist in predicting contamination events and optimizing cleaning cycle efforts.
Benefits of Using Statistical Tools
By implementing these statistical tools in your cleanroom monitoring program, you can expect the following benefits:
- Predict contamination events before they occur
- Optimize cleaning/disinfection cycles
- Improve root cause analysis (RCA)
Enhancing Cleanroom Monitoring with Particulate Size Analysis
In addition to using statistical tools, analyzing particulate size (in microns) and monitoring airborne particles in different states (At-Rest, Operational) can further enhance your cleanroom monitoring program.
This approach allows you to gain insights into the types and sizes of particles present in the cleanroom environment, enabling you to take targeted actions for contamination control.
Ensuring Compliance with Regulatory Guidelines
When it comes to aseptic processing facilities and environmental monitoring, it's essential to adhere to regulatory guidelines. One important regulation to consider is FDA 21 CFR Part 211, which outlines requirements for manufacturing practices.
By familiarizing yourself with these guidelines and incorporating them into your cleanroom operations, you can ensure compliance and maintain the highest standards of quality.
The Role of Climate Control and Water System Monitoring
In pharmaceutical manufacturing, maintaining product integrity and safety is paramount. Two critical aspects that contribute to this are climate control monitoring and water system microbial monitoring.
- Climate control monitoring ensures that temperature and humidity levels within the cleanroom are maintained as per specified requirements.
- Water system microbial monitoring helps detect any potential sources of contamination in the water supply used for cleaning or production processes.
By implementing robust monitoring protocols for both climate control and water systems, you can mitigate risks associated with product quality and safety.
Responding to Excursions
1. Immediate Actions for Contamination Events
- Quarantine impacted batch
- Resample affected area
- Notify QA and production
These immediate actions are crucial in the response to EM excursions to ensure that microbial and particulate levels monitoring is effectively conducted.
2. Root Cause Analysis (RCA)
- Use root cause analysis techniques such as 5 Whys or fishbone diagram
- Check HVAC logs, gowning logs, and EM equipment calibration
When conducting RCA, consider factors like particle size measurement in cleanrooms and explore methods to measure and control airborne particles in controlled environments.
3. CAPA Implementation
- Increase sampling frequency
- Retrain personnel within cleanrooms
- Revise SOPs
Effective corrective and preventive actions (CAPA) are essential for maintaining compliance with Cleanroom Classifications and guidelines set by organizations like GSA - U.S. General Services Administration and CEN - Committee for European Normalization.
Cleanroom EM Documentation Checklist
- Sampling plan records (zones, frequency, media)
- Equipment calibration documentation
- Growth promotion test results (GPT)
- Excursion logs and RCA with CAPA
- EM trend reports (monthly/quarterly)
- EM SOPs (sampling, data review, deviation handling)
📋 Tip: During regulatory inspection preparedness, regulators often start with your EM trend charts.
This cleanroom EM documentation checklist is essential for maintaining compliance with industry standards such as EU GMP Grades A to D. Ensure that your sampling plan records are thorough and that equipment calibration documentation is up to date. Regularly review growth promotion test results to verify the efficacy of your microbiological media.
When documenting excursions, make sure that excursion logs and RCA are detailed and include corrective actions as part of your CAPA process. Consistent EM trend reports will help you identify patterns and ensure that you’re effectively monitoring environmental conditions in the cleanroom.
Remember to adhere to cleanroom zoning and classification guidelines and consider the differences in Cleanroom Classification between FS 209E and ISO standards for cleanrooms and their applications in the pharmaceutical industry. Additionally, pay attention to humidity control and implement continuous particle and viable air monitoring in Grade A areas.
By following this checklist, you’ll be better equipped to learn how to monitor total particle counts and viable particles effectively, ensuring compliance and product safety standards.
Common Pitfalls to Avoid in Environmental Monitoring
- Treating excursions as isolated events, leading to excursion mismanagement
- Using expired media, which highlights the risks of expired media usage
- Inconsistent gowning before sampling due to inconsistent gowning practices
- Manual data entry errors, such as data entry errors in EM
- Not escalating borderline data trends in accordance with FDA aseptic manufacturing guidelines
Case Snapshot: How EM Saved a Sterile Facility
A sterile ophthalmic facility in India noticed a sudden increase in viable air counts in its ISO 7 staging area, raising concerns about cleanroom compliance. This incident prompted the EM team to quickly implement contamination prevention strategies by halting operations. Analyzing the situation through root cause analysis (RCA) revealed that a clogged HEPA filter was compromising air quality and that there was a lack of understanding regarding the importance of following the proper gowning sequence.
Timely intervention not only prevented contamination of a high-value product batch worth $2.1 million but also highlighted the critical role of HEPA filter maintenance in cleanrooms. This case study showcases the success of cleanroom EM and emphasizes the importance of understanding the requirements for ongoing monitoring and testing to maintain cleanroom compliance, particularly in accordance with ISO 14644-1 and ISO 14644-2 standards set by ISO Technical Committee 209.
Effective personnel monitoring and rigorous aseptic process simulation (APS) are essential practices in pharmaceutical facility design, especially within aseptic processing facility monitoring frameworks. Furthermore, it is crucial to comprehend sampling methods in cleanrooms to meet regulatory requirements for environmental monitoring in cleanrooms (GMP).
Future of Cleanroom EM Technology: Smarter, Faster, Predictive
- Real-Time Monitoring Solutions: Continuous particle and microbial sensors for effective environmental monitoring in cleanrooms
- AI in Environmental Monitoring: Predictive modeling for contamination risk, addressing microbial contamination and identifying particle contamination sources
- Wireless EM Devices: Reduced need for manual entry, streamlining total particle count monitoring in cleanrooms
- Cloud-Based EM Dashboards: Centralized control and faster audits, essential for compliance with EU Annex 1 standards
As the pharmaceutical industry moves toward Pharma 4.0 and EM evolution, environmental monitoring cleanroom practices will become more predictive and less reactive, ensuring higher standards of quality and safety.
Conclusion:
Cleanrooms are not static—they breathe, shift, and interact with people and processes. This dynamic nature makes environmental monitoring as a continuous process essential for tracking the invisible ecosystem of cleanroom EM culture shift.
Whether you're managing a sterile oncology facility or a nutraceutical softgel line, a strong EM program ensures that every breath of cleanroom air supports product safety assurance in cleanrooms and reinforces patient trust and EM compliance. In the race to meet cleanroom standards, EM isn't just your checklist—it's your compass.
Understanding Cleanroom Occupancy States (As-Built) and adhering to cleanroom classification standards like FS 209E cleanroom classes are crucial for identifying cleanroom contamination sources effectively. Additionally, employing viable particle monitoring techniques such as Tryptic Soy Broth (TSB) methods ensures that environmental monitoring remains robust and reliable.
As you navigate the complexities of cleanroom Water Systems in Pharmaceuticals, remember the importance of aseptic process simulation (media fill) testing for validating aseptic manufacturing processes.
FAQs
Q1. What is Environmental Monitoring (EM) in cleanroom sterile manufacturing?
Environmental Monitoring (EM) is the systematic collection and analysis of data from cleanroom environments to detect microbial and particulate contamination. It ensures sterility assurance, compliance with GMP/ISO standards, early contamination risk detection, and provides valuable trend analysis data.
Q2. What are the key components of a cleanroom EM program?
A comprehensive cleanroom EM program includes viable monitoring (sampling and culturing microorganisms), non-viable monitoring (airborne particle detection), personnel monitoring (assessing microbial shedding), and surface/equipment monitoring to maintain sterility and prevent contamination.
Q3. How should excursions or contamination events be managed in an EM program?
Excursion response involves immediate actions such as batch quarantine, resampling, and notification. Root Cause Analysis (RCA) methods like 5 Whys or fishbone diagrams are used alongside reviewing HVAC and gowning logs. Corrective and Preventive Actions (CAPA) may include increasing sampling frequency, personnel retraining, or revising SOPs to prevent recurrence.
Q4. What are common pitfalls to avoid in Environmental Monitoring for cleanrooms?
Common pitfalls include treating excursions as isolated incidents without trend analysis, using expired media for sampling, inconsistent gowning practices, manual data entry errors, and failing to escalate borderline data trends which can compromise sterility assurance.
Q5. Is automated EM better than manual?
Automated systems reduce error and offer real-time data, supporting faster investigations compared to traditional methods. When considering automated vs manual EM systems, it's important to note that automated systems can also integrate data on climate control parameters in cleanrooms more efficiently.
Q6. How does trending data support contamination control in cleanrooms?
Trending tools such as moving averages, box plots, and control charts help predict contamination events, optimize cleaning cycles, and improve root cause analysis by identifying patterns over time within environmental monitoring data.
Q7. What advancements are shaping the future of Environmental Monitoring in pharmaceutical cleanrooms?
The future of EM involves real-time monitoring with continuous sensors, integration of AI for predictive analytics, wireless devices for flexible data collection, and cloud-based dashboards enabling Pharma 4.0 compliance to enhance air safety and patient trust.




