by Udaykumar K. Rakibe, M.Pharm. Ph.D. MBA, Dr. Ajaz Hussain, Mr. Ram Balani
6 minutes
Navigating Regulatory Challenges in Pharmaceutical Manufacturing: Strategies for Global Compliance
How pharma can meet global regulations using AI-driven CAPA, quality culture, and harmonized SOP strategies.

Abstract
Pharmaceutical manufacturing operates within a dynamic regulatory landscape where adaptation to evolving global standards is essential. This article examines the interplay between FDA, EMA, WHO, and ICH frameworks while unpacking persistent challenges—data integrity lapses, supply chain opacity, and cost pressures. Drawing on familiar FDA 483 and Warning Letter findings, we propose risk-informed Corrective and Preventive Action (CAPA) strategies and explore how technological innovation such as generative AI/ML with RAG (Retrieval Augmented Generation), proactive governance, and harmonized systems can foster sustained regulatory excellence.
Introduction
The pharmaceutical industry's mission to deliver safe, effective, and high-quality medicines rests on its ability to navigate complex regulations. Agencies such as the FDA, EMA, WHO, and ICH continually update their standards to address emerging technologies, including AI-driven processes, placing manufacturers under increasing pressure to adapt. Challenges like maintaining data integrity, overseeing global supply chains, and managing costs threaten compliance, with failures risking regulatory penalties, financial losses, and reputational damage. This article examines these challenges, identifies common pitfalls, and provides transformative strategies to ensure regulatory alignment and operational resilience.
Consider noting that rapid innovation (e.g., AI) may outpace regulators’ capacity to update standards—posing a systemic challenge.
The Global Regulatory Landscape
Pharmaceutical manufacturers must comply with diverse standards set by key regulatory bodies:
- FDA (U.S.): Enforces 21 CFR regulations and Good Manufacturing Practices (GMP). The US FDA sets the global standard for published regulatory adherence by way of comprehensive datasets that include (a) Guidance PDFs and (b) 21 CFRs for mandatory compliance. However, its website search are meta-data based and outdated imposing even more challenges for all drug industry stakeholders including drugs manufacturers...
- EMA (EU): Oversees compliance through EU GMP directives.
- WHO and ICH: Provide harmonized guidelines for international markets.
- Harmonization initiatives like ICH Q12 still face implementation challenges.
These frameworks often diverge in focus—e.g., the FDA prioritizes data integrity, while the EMA emphasizes risk management—creating operational complexity. Requirements for documentation, quality control, and process validation further complicate compliance, especially as regulations evolve to address innovations like biologics and digital manufacturing. Staying ahead requires agility and tailored strategies for each region.
Key Compliance Challenges
Manufacturers face interconnected obstacles in achieving global compliance:
- Evolving Regulations: Frequent updates demand constant monitoring and swift adaptation to new standards.
- Quality Control Demands: GMP validation, testing, and documentation requirements strain resources and timelines.
- Supply Chain Vulnerabilities: Oversight of third-party vendors and raw material sourcing requires transparency and robust controls.
- Data Integrity Risks: Digital systems must ensure secure audit trails and prevent breaches, a growing concern in FDA inspections.
- Cost Pressures: Investments in compliance must be balanced with profitability, often under tight budgets.
- Cultural or organizational inertia.
These challenges amplify one another—evolving regulations, for instance, intensify quality control demands, while supply chain weaknesses heighten data integrity risks—necessitating a holistic approach.
Common Regulatory Pitfalls: Lessons from FDA 483s and Warning Letters
FDA inspections frequently cite recurring issues, highlighting systemic vulnerabilities:
- Data Integrity Failures: Incomplete audit trails or falsified records (21 CFR 211.68, EU GMP Chapter 4) undermine trust and safety.
- Inadequate Investigations: Weak deviation analyses (21 CFR 211.192, ICH Q10) delay root cause resolution, risking product quality.
- Contamination Risks: Poor sanitation practices (21 CFR 211.67) pose a risk to sterility and patient safety.
- Weak Quality Control Unit (QCU) Oversight: Typically, limited authority leads to unresolved batch issues and compliance gaps.
- SOP Non-Compliance: Lapses in aseptic procedures or training erode operational integrity.
- Illusion of control when systems are digitized but poorly validated.
These pitfalls often stem from outdated systems or insufficient resources, making them preventable with targeted action.
Systemic CAPA Reforms: Moving from Remediation to Maturity
To overcome these challenges, manufacturers can adopt the following CAPA strategies, bolstered by practical examples:
Data Integrity Modernization
- Example: A firm addressed FDA citations by implementing a validated Laboratory Information Management System (LIMS), ensuring traceable audit trails as required by 21 CFR 211.68.
- CAPA: Deploy electronic systems with role-based access and conduct quarterly integrity audits.
- Global Alignment: Standardize data policies and integrate AI-driven Quality Management Systems (QMS) for real-time oversight and control.
- SOPs, when cited as sources of deficiencies, partially occur because the manufacturer’s written guidelines or instructions fail to align with relevant 21 CFRs regulations. The FDA published 21 CFRs as the ‘what’ FDA requires, while an enterprise presumably documented the ‘how’ for SOP enforcement.
- A clear alignment between CFRs and SOPs imposes a large challenge given the massive textual information published by the FDA on one hand and an enterprise’s written SOP documentations in the other. For example, how does an enterprise ensure its SOPs with labeling align with 21 CFR 211 regulations wherever written documentations are mandatory and explicitly required by FDA? A purely human manual review of all 21 CFR 211 clauses for alignment becomes all too complex and error-prone with missed statutes.
- A tool such as eSTARHelper’s AI/ML-based Copilot, grounded on the FDA website FDA.Gov, integrated with SmartSearch+ full text search can greatly assist in filling the gap.
- The example below in Figure 1 illustrates a search for 21 CFR 211 clauses published by FDA, where written records are deemed mandatory in the entire drug manufacturing life cycle
- Clearly, though GenAI correctly identifies 21 CFR Part 211.192, GenAI prompting when used alone misses 21 CFR 204, as shown missing in Figure 1.
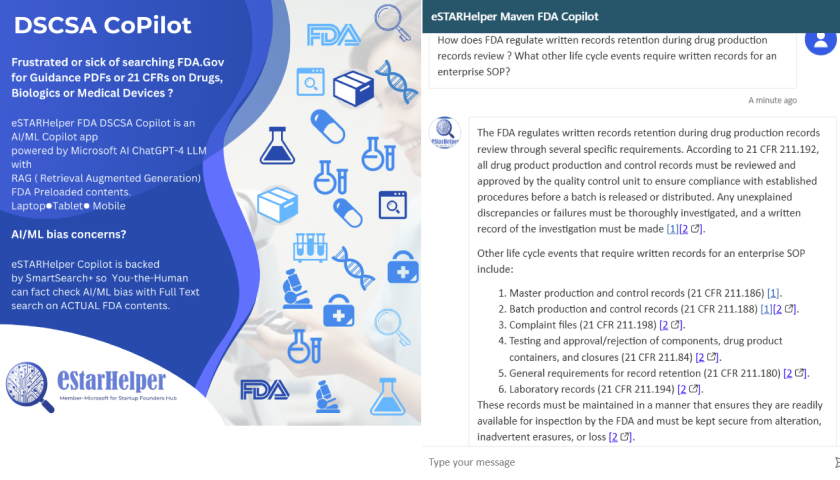
Figure 1: AI/ML FDA/Gov Copilot Generative ChatGPT-4o
Figure 2 below, however, shows written label records as required for returnable drugs when Human-in-the-loop with full-text SmartSearch+ is used.
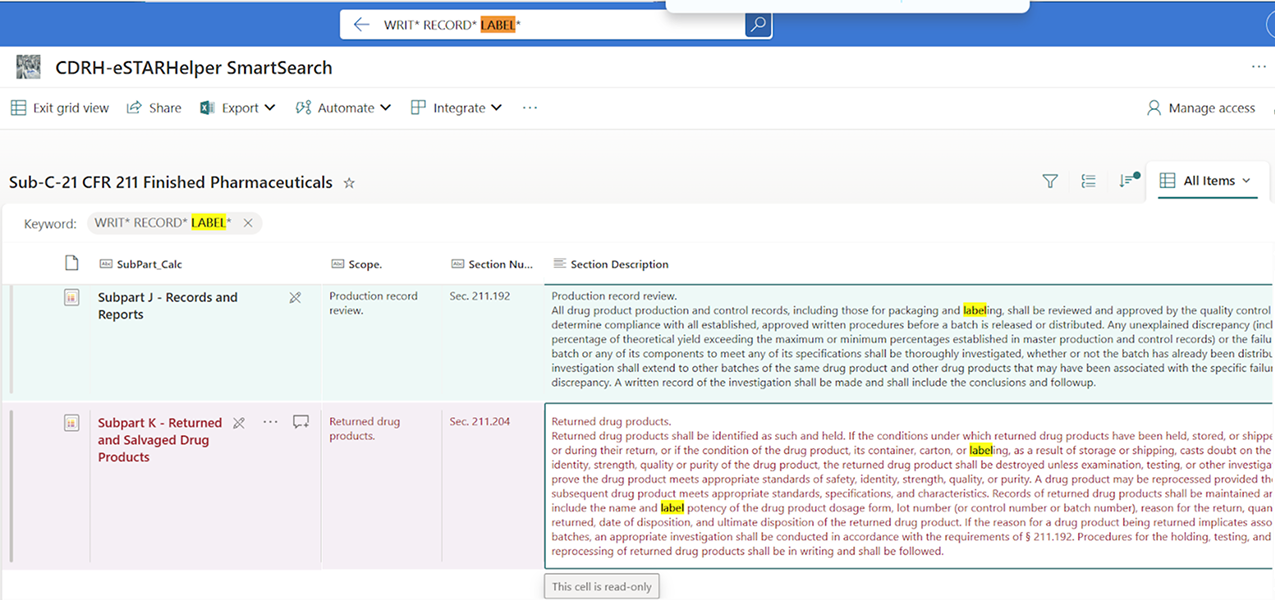
Figure 2: SmartSearch+ on 21 CFR 211 for Written record statutes related to labeling as a sample use case.
Robust OOS Management
- Example: Training in root cause analysis and a centralized tracking system cut Out-of-Specification (OOS) results by 30% at one manufacturer.
- CAPA: Utilize risk-based investigation frameworks and establish a global Out-of-Specification (OOS) database for shared learning.
- Global Alignment: Harmonize deviation protocols across regions for consistency.
Contamination Control
- Example: A sterile facility eliminated microbial risks by enhancing its standard operating procedures (SOPs) and validating disinfection methods.
- CAPA: Regularly validate cleaning procedures and perform cross-site contamination audits.
- Global Alignment: Standardize controls and share best practices via a centralized hub.
Empowering Quality Units
- Example: Automating QCU workflows reduced batch review failures by improving oversight efficiency.
- CAPA: Centralize QCU monitoring with real-time tools and grant greater decision-making authority.
- Global Alignment: Establish a global QCU network to standardize practices and share best practices and insights.
SOP Adherence
- Example: Surprise audits and updated training reduced SOP deviations by 25% at a manufacturing site.
- CAPA: Digitize SOPs for accessibility and launch global competency assessments.
- Global Alignment: Utilize digital platforms to monitor compliance and disseminate training resources.
- Traditional CAPA
- The traditional CAPA tends to be reactive and transactional—what’s needed is systemic, predictive capability.
- Enabling the investigation to identify the most probable root cause, and corrections, CAPA
- Facilitate the effectiveness strategy and prevention of repeats, by proactive integrating ICH Q10 and Q12 is desired.
Leadership's Role in Sustainable Compliance
Sustainable compliance hinges on leadership driving a quality-focused culture:
- Strategic Governance: Integrate regulatory priorities into corporate objectives, ensuring compliance remains the board's key focus.
- Resource Investment: Fund technologies like IoT for supply chain visibility and ongoing staff training.
- Risk-Based Decision-Making: Allocate resources to high-priority risks, such as data integrity or contamination.
- Quality Culture: Empower QCUs with autonomy to enforce standards without interference.
- Global Harmonization: Standardize practices across sites to streamline efforts and ensure consistency.
Leadership transforms compliance from a checkbox exercise into a driver of operational excellence and trust. When leadership is misaligned with quality objectives, even strong SOPs and systems fail.
Conclusion
Mastering pharmaceutical regulations demands more than meeting minimum standards—it requires proactive innovation and a steadfast commitment to leadership. By leveraging advanced technologies, implementing standardized CAPA frameworks, and fostering a culture of quality, manufacturers can turn regulatory challenges into opportunities for growth. Beyond avoiding penalties, these strategies ensure the reliable delivery of life-saving therapies, reinforcing the industry's role as a cornerstone of global health. In an ever-evolving landscape, compliance is not just a necessity, it's a competitive edge.
Compliance must evolve from a defensive necessity into a strategic driver of quality, innovation, and trust—anything less risks systemic failure in an increasingly interconnected world.
Comment your thoughts
Udaykumar Rakibe
looking for the inputs from the readers
8 months ago
Goutam Saha
It's an excellent article indeed, nicely laminated by focusing the industry needs churned with the experience of the stalwarts.
8 months ago
Laxmikant Harsola
Very nice summarized useful information
8 months ago
ghanshyam chaudhari
Very useful information
8 months ago
ghanshyam chaudhari
Great
8 months ago




