by Mrudula Kulkarni
7 minutes
Sterility at the Source – Utilities & SUS in Contamination Control Strategy (CCS)
Discover how utilities and SUS form the backbone of sterility and CCS in pharma.

In today’s pharmaceutical landscape, sterility is more than a regulatory requirement — it’s a business safeguard. One contaminated batch can mean millions of dollars in losses, supply disruptions, and reputational damage that takes years to recover from. For companies producing sterile injectables, biologics, vaccines, or ATMPs, the margin for error is razor-thin.
At the heart of sterility lies a robust Contamination Control Strategy (CCS) — the framework regulators expect every facility to demonstrate. While cleanroom classifications, gowning, and EM programs often take the spotlight, the less glamorous but absolutely make-or-break elements are the utilities and single-use systems (SUS) that support sterile manufacturing.
These are the true “source points” of contamination risk — if compromised, even the best cleanroom can’t compensate. In this pillar article, we’ll unpack how utilities (like clean steam, WFI, compressed air, and HVAC) and SUS (tubing, bags, connectors) form the backbone of sterility at the source, and how pharma leaders can strengthen their CCS by focusing here first.
1.CCS: The New Standard for Global Pharma
What CCS Really Means?
Contamination Control Strategy (CCS), as formalized in EU GMP Annex 1, is no longer just a compliance requirement—it’s a mindset shift. CCS compels pharma companies to move beyond individual controls and instead adopt a holistic, facility-wide approach that integrates risk assessment, environmental monitoring, cleaning, gowning, personnel behavior, and supply chain considerations into one unified framework.
From Reactive to Proactive Sterility
Traditionally, sterility testing was the safety net: detect contamination after the fact. CCS flips that model. The focus is now on proactive sterility assurance—designing facilities, processes, and behaviors so contamination never occurs in the first place. This means embedding real-time monitoring, predictive analytics, and closed-system processing into daily operations.
Why It Matters for Business?
Beyond regulatory compliance, CCS has tangible commercial value. Each contamination event or recall can cost millions—not only in product loss but also in delayed approvals, supply chain disruptions, and reputational damage. By embracing CCS, companies can achieve “zero recalls = zero regulatory disruptions”, translating into uninterrupted operations, market trust, and long-term competitive advantage.
2. Utilities: Hidden Risks, Visible Returns
Behind every aseptic operation lies a network of utilities—water, steam, gas, and air—that rarely make headlines but often decide the difference between compliance and crisis. These “silent enablers” are also where smart investments deliver some of the highest long-term returns.
2.1 Purified Water (PW) & WFI Loops
Water systems are the lifeblood of pharma manufacturing. But when biofilm takes root in a loop, the costs go beyond remediation chemicals and downtime—they extend to batch loss, regulatory citations, and reputational damage.
- The Risk: Biofilm outbreaks can force plant shutdowns and trigger product recalls.
- The Fix: Continuous sanitization (ozone, hot water >65 °C) combined with online monitoring for conductivity, TOC, and microbial load.
ROI: Faster deviation detection, fewer excursions, and extended loop life translate to measurable savings and stronger compliance assurance.

2.2 Clean Steam & Sterile Gas Systems
Contaminated clean steam or gases can compromise sterilizers, reactors, and isolators—introducing invisible risks that only become visible after product failure.
- The Risk: A single contamination event can halt production, causing weeks of downtime.
- The Fix: Validated filtration systems and redundancy designs ensure continuity and sterility even during filter failures or maintenance cycles.
- ROI: The cost of dual filters or periodic validation is negligible compared to lost revenue from halted operations.
2.3 HVAC & Air Systems
Air systems represent one of the most complex and costly utilities to manage. Yet, contamination excursions due to poor airflow or filtration are far costlier than preventive maintenance.
- The Risk: Airborne contamination can lead to facility shutdowns, failed EM results, and rejected product.
- The Fix: Proactive HVAC maintenance with smart monitoring systems that track pressure differentials, particulate load, and filter performance in real time.
ROI: Beyond compliance, smart HVAC controls optimize energy use, reducing OPEX while safeguarding sterility assurance.
3. Single-Use Systems (SUS): Risk or Revenue Advantage?
The rise of biologics and personalized therapies has accelerated the adoption of Single-Use Systems (SUS), with nearly 70% of new biologics manufacturing facilities now integrating SUS into their operations. The appeal is clear: faster batch turnover, reduced cleaning validation, and quicker revenue realization. By eliminating time-intensive cleaning and sterilization cycles, SUS accelerates time-to-market while lowering utility and labor costs.
But concerns remain—particularly around sterility and leachables. These risks are increasingly mitigated through validated SUS suppliers, robust quality agreements, and closed transfer systems that minimize operator intervention. For manufacturers, the decision often comes down to this: treat SUS as a compliance risk or as a strategic revenue accelerator. The companies that succeed are those that invest in qualified suppliers, strong documentation, and lifecycle management from day one.
Checklist: Choosing a SUS Partner That Protects Your Product
- Supplier certifications (ISO 11137, USP <87>/<88>, extractables/leachables studies).
- Proven sterility assurance—gamma irradiation or X-ray sterilization validation.
- Scalability of SUS components across clinical → commercial production.
- Supply chain resilience (dual sourcing, inventory buffers).
- Compatibility with closed-system processing and automation platforms.
- Transparent documentation and audit readiness.
4. Utilities + SUS: The CCS Profit Equation
When utilities and Single-Use Systems (SUS) are perfectly aligned, the result is more than operational efficiency—it’s audit-ready facilities, minimal downtime, and, ultimately, patient trust.
Take Company X as an example. By investing in a robust Purified Water (PW) loop and a validated SUS supply chain, they avoided a $50M product recall. That’s not just savings; that’s reputation preserved and patients protected.
📊 Flowchart: How Strong CCS Saves You Money
- Optimized Utilities → Reliable Processes → Fewer Batch Failures
- Validated SUS → Faster Batch Turnover → Reduced Risk of Contamination
- Combined CCS Strength → Audit-Ready Facility → Patient & Investor Confidence
- Result → Lower Operational Costs + Protected Revenue
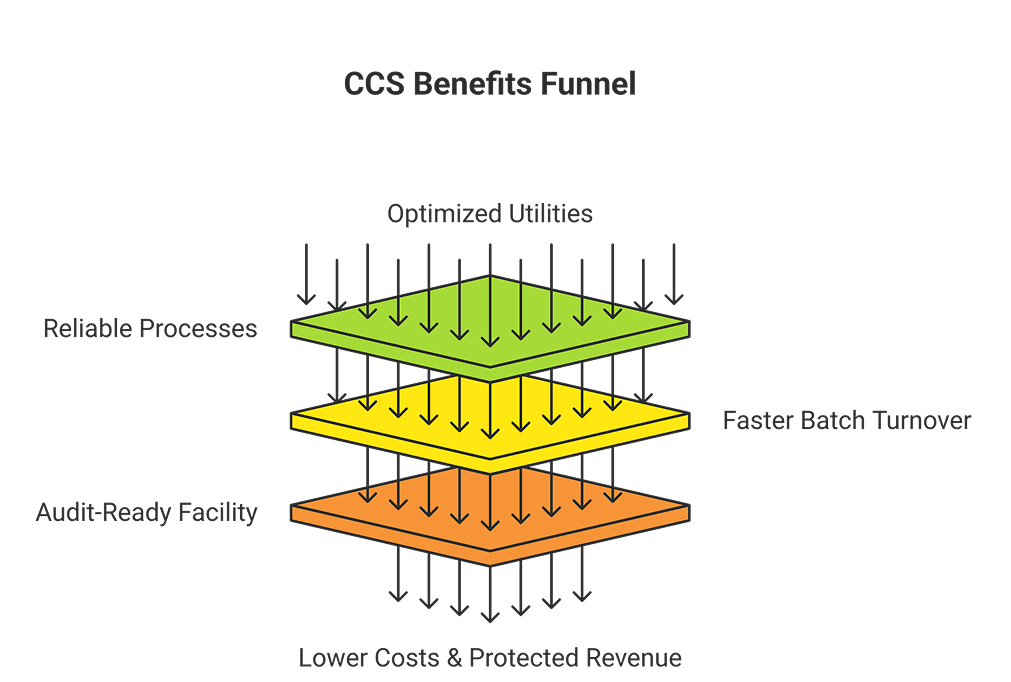
Bottom line: Utilities + SUS isn’t just a technical alignment—it’s a profit strategy for modern pharma facilities.
5. Regulatory & Market Differentiation
Regulators—FDA, EMA, MHRA—don’t just check boxes. They expect proof of sterility assurance right at the source.
Pharma companies that go beyond baseline compliance don’t just avoid warning letters—they earn trust and become preferred CDMO partners. Excellence becomes a market signal: higher credibility, stronger partnerships, and a competitive edge.
Message: Compliance is mandatory. Excellence is your differentiator.
Step 1: Meet Regulatory Baseline
- Follow GMP and sterility requirements
- Maintain auditable records for FDA, EMA, MHRA
Step 2: Validate at the Source
- Sterility assurance built into every process
- Automated monitoring + real-time QC checks
Step 3: Go Beyond Compliance
- Implement advanced environmental controls
- Continuous improvement in process reliability
Step 4: Earn Market Trust
- Become a preferred CDMO partner
- Build credibility with clients and regulators
Outcome:
✅ Audit-ready facilities
✅ Reduced risk of recalls or inspection issues
✅ Competitive edge through operational excellence
The pharma landscape is evolving fast. Staying compliant is no longer enough—leading the conversation on sterility and process excellence is what separates the front-runners from the followers.
6. Future-Proof Your Facility
The pharma landscape is evolving fast. Staying compliant is no longer enough—leading the conversation on sterility and process excellence is what separates the front-runners from the followers.
Emerging Trends:
- AI-Powered Monitoring: Artificial intelligence now enables predictive maintenance, anomaly detection, and automated quality checks. By identifying potential process deviations before they impact product sterility, AI reduces downtime, prevents batch losses, and strengthens regulatory confidence.
- IoT-Enabled Utilities: Sensors embedded across water loops, HVAC systems, and cleanroom utilities deliver real-time data on temperature, pressure, and contamination risks. Continuous monitoring allows for immediate corrective action, improving both operational efficiency and audit readiness.
- Hybrid Single-Use Systems (SUS): Combining disposable components with traditional stainless-steel infrastructure creates flexibility without compromising control. This hybrid approach supports faster batch turnover, reduces cleaning validation requirements, and minimizes the risk of cross-contamination.
The Advantage of Early Adoption:
Companies that invest in these technologies ahead of the curve don’t just optimize operations—they gain a first-mover advantage:
- Cost Savings: Reduced downtime, lower maintenance expenses, and minimized batch losses translate directly to improved margins.
- Audit Credibility: Demonstrating proactive adoption of cutting-edge monitoring systems signals operational excellence to regulators and clients alike.
- Market Differentiation: Leading the sterility conversation positions your facility as a trusted, innovative partner for CDMOs, biopharma companies, and investors.
“Don’t just follow regulations. Lead the sterility conversation.”
Conclusion: From Cost Center to Value Driver
Utilities and Single-Use Systems (SUS) are often seen as operational burdens—line items that drain budgets and demand constant attention. But the perspective shift is simple: treat them as investments in sterility insurance.
When designed, monitored, and validated effectively:
- Utilities become reliable workhorses that ensure water, air, and energy consistently meet quality standards.
- SUS reduces contamination risk, accelerates batch turnover, and protects your product at every stage.
Together, they transform a traditional cost center into a strategic value driver—one that safeguards patient safety, enhances audit readiness, and builds trust with regulators and clients.
“Your next audit shouldn’t test sterility. Your systems should prove it.”
FAQs
- How can strong CCS reduce recall risks?
- A robust utilities and SUS framework ensures sterility is maintained from the source to the final product. Continuous monitoring, validated SUS supply chains, and reliable water/air systems minimize contamination risks, preventing batch failures and costly recalls. In short: strong CCS proactively protects patients and revenue.
- What’s the ROI of upgrading a PW/WFI loop?
- Upgrading a Purified Water (PW) or Water for Injection (WFI) loop isn’t just capex—it’s a risk-mitigation investment. Benefits include fewer batch failures, reduced downtime, lower maintenance costs, and audit readiness. Companies often see ROI through avoided recalls, operational efficiency, and regulatory confidence within the first few production cycles.
- Are SUS really more cost-effective than stainless steel long-term?
- Yes, when deployed strategically. Single-Use Systems (SUS) reduce cleaning validation, minimize cross-contamination risk, and allow faster batch turnover. While initial costs can be higher, long-term savings in labor, downtime, and risk management often outweigh stainless steel investments, especially for biologics and multi-product facilities.
- How do regulators view modern monitoring tools in CCS?
- Regulators like FDA, EMA, and MHRA increasingly favor facilities that integrate AI, IoT, and continuous monitoring into their utilities and SUS. Demonstrating real-time control, predictive maintenance, and automated audit trails strengthens compliance and shows leadership in sterility assurance.
Can investing in CCS give my facility a competitive advantage in CDMO partnerships?
Absolutely. Facilities that go beyond baseline compliance and demonstrate advanced utilities + SUS integration are viewed as trusted, high-quality partners. This positions them as preferred CDMOs, attracting clients who prioritize reliability, speed, and sterility assurance.





