by Mrudula Kulkarni
7 minutes
How to Ensure Pharmaceutical Safety Through Endotoxin Lifecycle Management and Depyrogenation Validation
Learn how ELM and depyrogenation protect patient safety and strengthen pharma compliance.
In pharmaceutical manufacturing, patient safety is paramount. Among the critical risks, endotoxins, also known as pyrogens, present a unique challenge. These heat-stable lipopolysaccharides, primarily from Gram-negative bacteria, can trigger severe febrile reactions and, in some cases, life-threatening complications if they contaminate injectable drugs, biologics, or medical devices.
“Endotoxin control is not just a regulatory requirement—it is a moral obligation to protect patients,” says experts in pharmaceutical quality assurance.
Implementing Endotoxin Lifecycle Management (ELM) alongside validated depyrogenation processes is therefore essential for robust endotoxin risk management and ensuring regulatory compliance.
What Are Endotoxins and Why Are They Dangerous?
Endotoxins are resilient molecules that can survive standard sterilization processes. Their presence in pharmaceuticals can lead to pyrogenic reactions, including fever, hypotension, and systemic inflammation. Even trace amounts can compromise patient safety, making endotoxin control in pharma critical.
Global regulatory agencies, including the US FDA, EMA, and ICH, emphasize proactive endotoxin management. USP <85> Bacterial Endotoxins Test and USP <71> Sterility Tests provide standardized frameworks for testing, while ICH Q7 guidelines reinforce preventive strategies.
“Compliance is only part of the story—our true goal is zero patient exposure to harmful endotoxins,” notes a quality assurance director.
What Is Endotoxin Lifecycle Management (ELM)?
Endotoxin Lifecycle Management is a comprehensive approach for preventing, monitoring, and mitigating endotoxin contamination throughout a pharmaceutical product’s lifecycle—from raw materials to the finished product.
Key steps in ELM include:
- Risk Assessment: Identifying all possible sources of endotoxin contamination, including water systems, equipment, and raw materials.
- Process Control: Implementing preventive measures such as validated depyrogenated glassware, sterile filtration, and monitored WFI systems.
- Monitoring and Testing: Regular LAL testing or recombinant Factor C assays for intermediates, water systems, and finished products.
- Corrective Actions: Root-cause analysis, batch quarantine, and remediation in case of detected endotoxins.
- Continuous Improvement: Using trend analysis and data-driven insights to enhance processes and prevent recurring contamination.
_-visualselection120251009143550.png)
“Knowing where the risk originates is the first step in preventing contamination,” says an industry expert.
Depyrogenation: The Cornerstone of Endotoxin Control
Depyrogenation is the critical process of removing or inactivating endotoxins from equipment, containers, and pharmaceutical products. Unlike sterilization, which eliminates microbes, depyrogenation specifically targets heat-stable endotoxins, ensuring patient safety and regulatory compliance.
Common methods include dry heat sterilization, where equipment or glassware is heated to 180–250°C for validated durations; steam sterilization, which is effective for microbes but requires specific validation to inactivate endotoxins; alkaline treatment, using caustic solutions for heat-sensitive materials; and filtration, employing endotoxin-retentive filters (0.2 μm) for liquids.
“Depyrogenation is the invisible shield that protects patients from unseen microbial toxins,” emphasizes a senior pharmacovigilance professional.
Depyrogenation Validation: Ensuring Consistency
Depyrogenation validation proves that the process consistently achieves required endotoxin reduction. Regulatory frameworks such as FDA 21 CFR Part 211 and EU GMP Annex 1 require documented evidence that all manufacturing systems, containers, and equipment are effectively depyrogenated.
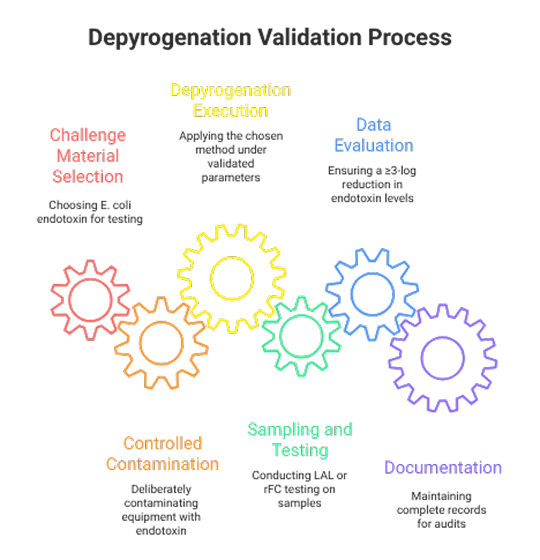
Steps in Depyrogenation Validation
- Challenge Material Selection: Typically, E. coli endotoxin is used.
- Controlled Contamination: Deliberate contamination of equipment with a known endotoxin load.
- Depyrogenation Execution: Applying the chosen method under validated parameters.
- Sampling and Testing: Post-process LAL or rFC testing of samples.
- Data Evaluation: Ensuring a ≥3-log reduction in endotoxin levels.
- Documentation: Complete records for audits, including raw data and deviations.
“Validation isn’t a one-time task; it’s a commitment to consistency and patient safety,” explains quality leaders.
Integrating ELM and Depyrogenation in Pharma Manufacturing: Challenges and Benefits
To ensure comprehensive endotoxin control, it is essential to combine Endotoxin Lifecycle Management (ELM) with validated depyrogenation processes throughout pharmaceutical manufacturing. This involves continuously monitoring and periodically depyrogenating Water for Injection (WFI) systems, subjecting glassware, tanks, and single-use systems to validated depyrogenation cycles, and incorporating endotoxin testing at critical points during process validation batches.
By integrating these strategies, organizations not only maintain regulatory compliance but also establish a culture of proactive patient safety. “When ELM and depyrogenation validation work together, the result is a culture of proactive patient protection,” notes an industry expert, emphasizing how these practices safeguard every product and reinforce trust in patient care.
However, implementing these processes comes with its challenges. Heat-sensitive materials and biologics can be difficult to depyrogenate without compromising product integrity. Complex single-use systems and advanced therapies, such as gene and cell therapies, introduce additional layers of risk due to their sensitivity and intricate manufacturing requirements.
To address these challenges, best practices include adopting a risk-based approach for critical components, employing real-time monitoring for water systems and products, maintaining detailed documentation to support regulatory audits, and fostering cross-functional collaboration across manufacturing, quality, and compliance teams to proactively mitigate endotoxin risks. By embracing these strategies, companies can not only overcome operational hurdles but also elevate patient safety as the central focus of their manufacturing culture.
The Future of Endotoxin Control
Pharmaceutical manufacturing is rapidly evolving, with AI-driven analytics and digitalization transforming how endotoxin risks are managed. Technologies such as digital twins of manufacturing systems enable virtual validation of depyrogenation processes, allowing manufacturers to anticipate potential contamination rather than merely reacting to it. “Technology is empowering us to anticipate risk rather than react to it,” notes a pharmacovigilance leader, highlighting how innovation is reshaping patient safety practices.
Endotoxin Lifecycle Management (ELM) combined with validated depyrogenation remains the cornerstone of safeguarding patients and ensuring regulatory compliance. By adopting a lifecycle-based approach, implementing validated depyrogenation cycles, and leveraging modern technologies, pharmaceutical companies can proactively monitor, control, and mitigate endotoxin contamination.
“Patient safety begins with proactive control. Every preventive measure we take today ensures tomorrow’s therapies are safe and reliable,” emphasize industry experts.
By integrating these strategies into their operations, manufacturers not only meet regulatory expectations but also establish a culture of proactive patient protection, ensuring that every product released is safe, effective, and trustworthy.
In the end, comprehensive endotoxin control is not just a regulatory requirement—it is a commitment to the patients whose lives depend on every therapeutic decision made today.
FAQs
1. What is endotoxin lifecycle management (ELM) in pharma?
Endotoxin Lifecycle Management (ELM) is a structured approach to prevent, monitor, control, and mitigate endotoxin contamination throughout a pharmaceutical product’s lifecycle—from raw materials to finished products.
2. Why is depyrogenation validation important?
Depyrogenation validation ensures that processes used to remove or inactivate endotoxins consistently achieve the required reduction, safeguarding patient safety and meeting regulatory compliance.
3. What are the common methods of depyrogenation?
Common depyrogenation methods include dry heat sterilization, steam sterilization (validated for endotoxins), alkaline treatment for heat-sensitive materials, and endotoxin-retentive filtration for liquids.
4. How is endotoxin contamination detected?
Endotoxins are commonly detected using LAL (Limulus Amebocyte Lysate) tests or Recombinant Factor C (rFC) assays, which are sensitive, validated methods for detecting trace endotoxin levels.
5. What are the key regulatory guidelines for endotoxin control?
Key guidelines include USP <85> Bacterial Endotoxins Test, USP <71> Sterility Tests, ICH Q7, FDA 21 CFR Part 211, and EU GMP Annex 1.




