by Ravindra Warang
12 minutes
Everything About Injectables & Liquid Filling: Manufacturing, Types & Trends
Deep dive into injectable drug manufacturing—from formulation to filling, sterility, compliance, and cutting-edge innovations.

Let's enter a place where silence is not just preferred but necessary. In a cleanroom for making injectable medicines, machines quietly work while a scientist, dressed in sterile gear, carefully picks up a vial from a fast filling line. This vial might hold a chemotherapy drug, a life-saving vaccine, or an antibiotic used in an ICU.
Every second matters here. Every milliliter is checked. Every surface is kept so clean that even a tiny speck of dust could stop production. This is the world of sterile manufacturing—the quiet force behind every injectable medicine you’ve ever had.
Unlike pills that go through your digestive system, injectables go straight into your bloodstream. There’s no filter and no time to waste. That’s why injectables must be perfect. Sterile manufacturing isn’t just about following steps—it’s about keeping trust, saving lives, and achieving precise drug delivery.
In this guide, we’ll look at how science and discipline shape injectable drug production—from the formulas and types to the amazing filling machines used today. We’ll explore aseptic fill-finish processes, new technologies like digital filling systems and Blow-Fill-Seal packaging for liquid medicines, and explain how regulations ensure safety. Think of this as your behind-the-scenes look at one of pharma’s most exacting and important areas. For a complete look at global innovations and leaders in this segment, explore our feature on top liquid filling machine manufacturers.
We’ll also cover different types of freeze-dried injectables and explain how prefilled syringe filling machines work. Join us to see how companies like Ajinomoto Bio-Pharma Services are raising the bar with advanced tools like high-viscosity fillers and volumetric filling machines featuring special 3-rib ETFE-coated syringe plungers.
Get ready to dive into a strict world where training staff and validating sterilization processes are key to making sure every injectable medicine is safe and effective.
1. What Are Injectable Preparations?
Picture being in an emergency room. A patient arrives, and a nurse quickly prepares an injection—maybe an antibiotic or painkiller. This single dose, drawn into a clean syringe, can save a life within minutes. It works fast because it goes straight into the body without needing to be digested or filtered by the stomach.
Injectable preparations, also called parenterals, are medicines made to be given directly into the body, avoiding the digestive system. This means the medicine reaches the bloodstream fully and immediately.
Because they go straight into the body, these injections must be perfectly clean. Even tiny amounts of bacteria or harmful substances can cause serious problems. That’s why injectable medicines are made carefully in sterile conditions—free from germs and particles—to ensure they are both safe and effective.
Injectables are essential tools in healthcare, used in places like intensive care units, maternity wards, cancer centers, and vaccination clinics. Dive deeper into the principles of sterile manufacturing in pharma to understand how injectable facilities maintain zero-contamination environments. Knowing how they are made helps us appreciate the science and care behind every shot.
As new treatments develop, companies like VisionGain lead in improving injectable manufacturing. They create advanced products like lipid nanoparticles (LNPs) that help deliver drugs better while following strict safety rules set by regulations such as 21 CFR parts 210 and 211.
When learning about rules for making injectables—like Annex 1 and cGMP guidelines—it’s important to focus on steps like process validation and monitoring clean environments. These steps make sure every part of making injections meets high-quality standards.
Technology has also improved how injectables are produced. Automatic filling machines with vacuum systems and fast vent tubes help speed up production while reducing contamination risks.
In short, injectable preparations play a vital role in modern medicine and require careful attention at every step—from making them to giving them to patients.
2. Types of Parenteral Preparations & Ways to Give Them
If you opened a fridge in a drug warehouse, you'd see not just vials but also ampoules, syringes, and small bottles with powders—each made for different medical needs. These are different kinds of injectable medicines used to deliver drugs effectively.
Some injectables are clear liquids, ready to use right away. Others are suspensions, where tiny particles float in sterile fluid to provide longer effects. Emulsions mix oil and water to help release drugs slowly. Lyophilized powders stay dry until mixed with liquid before injection—good for vaccines that need careful storage. Implantable gels release medicine slowly over weeks or months.
The type chosen depends on the drug’s nature, how stable it is, where it needs to work, and patient needs. For example:
- Solutions work well for quick effects throughout the body.
- Suspensions are good for slow-release injections.
- Emulsions help with drugs that don’t dissolve well.
- Lyophilized powders last longer on shelves for sensitive drugs.
Now, about how these medicines are given. The body has several injection routes:
- Intravenous (IV): Into the bloodstream—fastest and most common.
- Intramuscular (IM): Into big muscles—used for vaccines and long-lasting drugs.
- Subcutaneous (SC): Under the skin—used for insulin and biological medicines.
- Intradermal (ID): Into the skin layer—used for allergy tests and TB shots.
- Intraperitoneal (IP): Less common in people but used in research.
Each route needs different needle sizes, amounts of medicine, and thickness of the drug. That’s why making injectables requires careful precision and cleanliness to keep them sterile and safe.
Behind every type is work by scientists making stable formulas, engineers designing safe equipment, and machines that perform the process perfectly every time. Quality checks like container sealing tests make sure the medicine stays good during production.
When learning about injectable drug making, it’s important to understand transferring technology between companies and choosing contract manufacturers for early testing through full production. Knowing FDA rules for sterile injectable production helps ensure safety and success in this complex field.
3. Types of Injectables in Pharmaceutical Manufacturing
In a pharmaceutical plant, you’ll find more than machines—you’ll see many types of medicines made to treat different health needs.
General Injectables
These are common medicines like vaccines, antibiotics, and painkillers given by injection. They look simple but must be made very clean and with exact doses. They work quickly, safely, and reliably for many patients.
Cytotoxic Injectables
These are strong cancer-fighting drugs that kill fast-growing cells. They can be dangerous to handle, so they are made in special clean rooms with strict safety rules and trained staff. Making these drugs requires very careful steps to ensure safety and effectiveness.
Freeze-Dried (Lyophilised) Injectables
These medicines are frozen and dried to keep them stable for a long time. This method helps important drugs like vaccines and hormones last through shipping and storage. Before use, they are mixed with sterile water to become liquid again.
Liquid Injectables
These ready-to-use liquid medicines work fast. Examples include emergency drugs like epinephrine and saline solutions. They are often filled using automatic machines to keep the process quick and sterile.
Powder Injectables
Some medicines are made as powders because they last longer this way. These powders are mixed with liquid just before giving the injection. This form is good for antibiotics and other drugs that don’t stay stable in liquid form. Special equipment is used to avoid contamination during production.
Beta-Lactam Injectables
These antibiotics come from penicillin and related drugs. They are strong but can cause allergies, so they are made in separate areas with advanced air filters to prevent contamination.
Non-Beta-Lactam Injectables
These antibiotics, like vancomycin, treat infections resistant to other drugs. Their production is careful but doesn’t need as strict isolation as beta-lactams, though cleanliness is still very important.
Each type of injectable medicine has been developed through years of research and safety checks. New technology like prefilled syringes and autoinjectors helps make drug delivery safer and more reliable for patients. The field of injectable medicine keeps improving to provide better treatments with less risk.
→Explore the injectable drug manufacturing trends shaping the future of fill-finish operations.
4. Sterile Manufacturing Process for Injectables
Every injectable goes through careful steps to make sure it’s safe and free from germs. Imagine a production area so clean and quiet that even breathing is controlled.
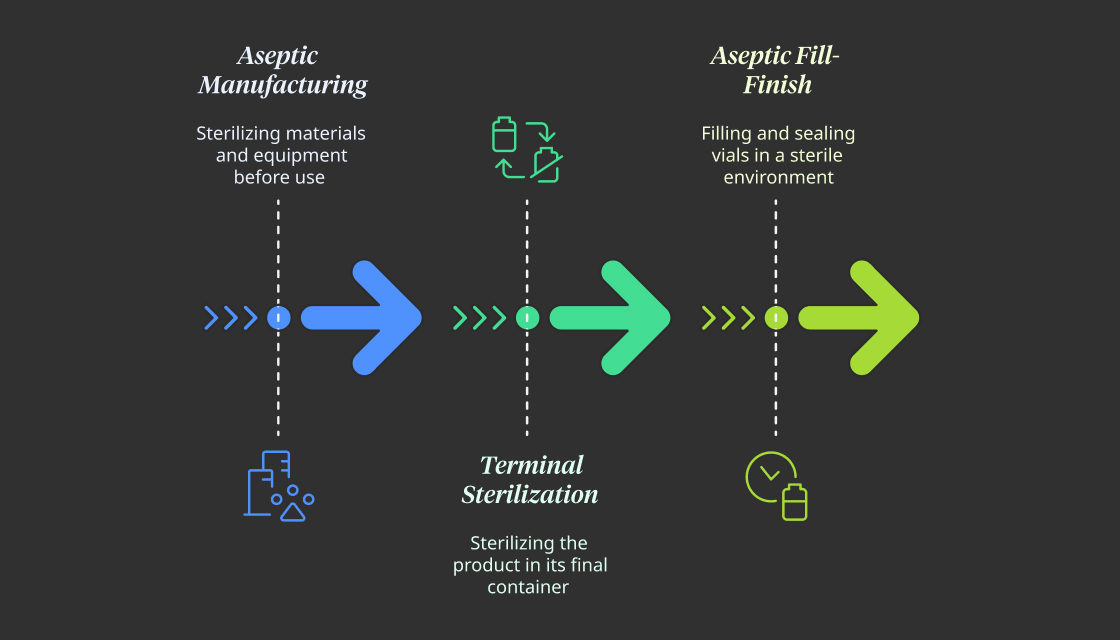
Aseptic Manufacturing
This method is used for injectables that can’t be sterilized with heat, like biologics, vaccines, and hormone drugs. All materials and equipment are sterilized before use. Workers operate inside super-clean rooms with special airflow systems to keep the environment sterile. It requires precise control and strict procedures.
Terminal Sterilization
When possible, this method is preferred because it’s safer and simpler. The product is placed in its final container—like a vial or syringe—and then sterilized using heat, steam, or radiation. This works well for stable drugs like small molecules, IV fluids, and some antibiotics. However, sensitive drugs can be damaged by heat or radiation, so this method isn’t always suitable.
Aseptic Fill-Finish
This step combines skill and technology. In a sterile room, machines fill vials with the drug solution and seal them immediately with sterile stoppers. Cameras monitor every move to catch even small errors. Technologies like Restricted Access Barrier Systems (RABS) and isolators help keep everything sterile while meeting strict FDA rules.
The choice of these methods depends on the drug’s properties and how it will be used. The main goal is clear: no germs, no risks.
Patients may never see these cleanrooms or machines, but their trust in each dose depends on the care taken during manufacturing.
For more details on sterile manufacturing standards, you can look into Annex 1 guidelines, which cover cleanroom design and environmental monitoring to ensure top-quality sterilization processes.
5. Injectable Filling: Importance & Setup
Imagine a ballet, but instead of dancers, machines, sensors, and sterile nozzles move perfectly together. This is what a fast injectable filling line looks like—a place where every step matters because lives depend on it.
Injectable filling isn’t just about putting liquid into glass containers—it requires precise engineering, cleanliness, and speed. Whether it’s insulin in syringes or cancer drugs in vials, this process connects the medicine with its packaging while making sure no contamination happens. This is especially important for sterile injectables, where any contamination can be very dangerous.
Every injectable filling line follows three main rules:
- Accuracy: Giving the exact dose, even down to tiny drops.
- Sterility: Keeping out all contaminants to keep the product safe.
- Efficiency: Working quickly without risking safety.
These lines have several connected parts:
- Machines that wash vials or ampoules using ultrasonic waves and strong jets.
- Sterilization tunnels that use dry heat to kill bacteria and toxins.
- Filling units with special pumps that measure doses precisely.
- Stoppering systems that immediately seal containers to keep them sterile.
- Capping, inspection, and labeling stations that prepare products for shipping.
The setup supporting these lines is also very strong:
- Cleanrooms with strict control of temperature, humidity, and airflow following cleanroom standards.
- HEPA filters that blow clean air to keep the environment free of particles.
- Systems that monitor pressure, particles, and microbes in real-time.
What makes a good filling line great is reducing human contact through automation. The cleaner the environment, the more the process uses advanced tech like robotic arms and vision systems. Electronic records track everything to meet FDA rules. Learn how digital twins are shaping pharma manufacturing by optimizing sterile filling lines through predictive control.
In injectable drug manufacturing, even one wrong drop or poor sealing can cause harm instead of healing. That’s why this setup isn’t just equipment—it’s a promise of safety in every product.
When learning about automated injectable drug production, remember how crucial contamination control is in sterile areas and follow best practices in staff training and microbiological testing. Whether using semi-automatic machines or advanced aseptic technology, every part helps ensure the medicine is safe and effective.
6. Types of Injectable Filling Machines
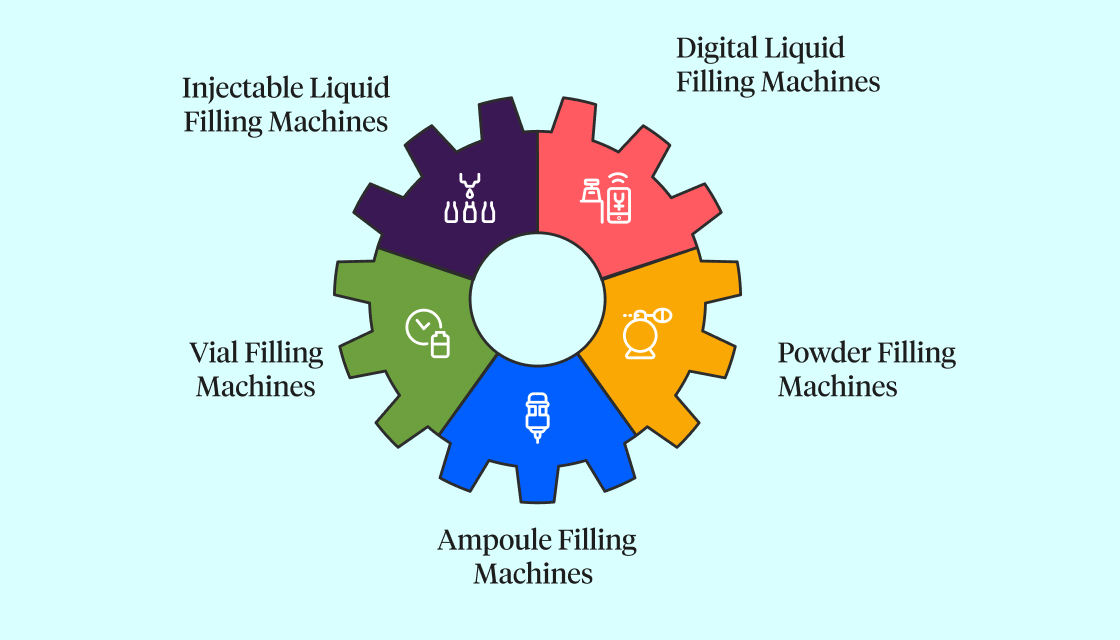
In injectable drug production, machines work precisely and continuously to fill containers safely and quickly.
6.1 Injectable Liquid Filling Machines
These machines fill vials with liquid by carefully balancing pressure to avoid spills or air bubbles. They use sensors to detect missing containers and adjust filling speed without needing mechanical changes. They are ideal for vaccines, saline solutions, and general medicines, with accuracy often within ±1%.
6.2 Digital Liquid Filling Machines
These advanced machines combine software and mechanics for smart control. They monitor each vial during filling, automatically adjusting if problems occur and stopping defective doses. They are perfect for high-speed production and modern pharmaceutical lines.
6.3 Vial Filling Machines
Small and efficient, vial filling machines are good for limited space and smaller production runs, like in biotech startups or labs. They check the weight of filled vials and reject any that don’t meet quality standards, using less energy while ensuring sterile conditions.
6.4 Powder Filling Machines
Designed for dry drugs, these machines quickly fill vials or ampoules with precise amounts of powder without waste or dust. They are used for freeze-dried drugs and temperature-sensitive products.
6.5 Ampoule Filling Machines
These machines fill small sealed glass containers (ampoules) and then flame-seal them to keep the contents sterile and safe from tampering. They handle sensitive medicines like cancer drugs or emergency kits with minimal contamination risk.
Choosing the right machine depends on the product type, speed needed, and sterility requirements. Find out which innovations are leading the way in our report on the top 10 pharmaceutical machineries in 2025. With smaller batches and more complex products today, flexibility, automation, and strict cleanliness are essential.
When selecting aseptic filling technology, make sure it meets regulations from authorities like Italy’s AIFA or the US CDER. Also, ensure your facility uses HEPA filters to control contamination and that staff are trained to work in sterile environments to keep injectable drugs safe throughout production.
7. How to Choose the Right Filling Line
Picking the right injectable filling line is like choosing the best vehicle for an important trip. You wouldn’t take a fast train through scenic mountains, and similarly, not every machine fits every drug. The best machines aren’t always the most expensive or fastest—they are the ones that match the drug’s needs perfectly.
So, how do pharmaceutical makers decide?
Product Features
Is the drug sensitive to heat or light? Is it thick, foamy, or full of particles? Knowing these details helps decide everything from filling speed to container type. For example, freeze-dried biologics like monoclonal antibodies need special clean environments and humidity control, while saline can be filled quickly with simple machines.
Container Type
Different containers—small ampoules, strong cartridges, or prefilled syringes—need different equipment. The container material (glass or plastic) also affects whether heat sterilization can be used.
Batch Size and Production Volume
Batch size and production levels matter. A small biotech making limited cancer drug batches will want compact, flexible machines. A vaccine maker supplying worldwide needs fast, scalable systems.
Automation Level
How much human work is allowed? For very strong or toxic drugs, fully automated systems in isolated areas are needed. Understand the full impact of robotics in pharmaceutical manufacturing in our in-depth market trend analysis. For regular injectables, partly automated lines may be enough.
Regulatory Rules
The machine must follow current Good Manufacturing Practices (cGMP), validation rules (IQ/OQ/PQ), and 21 CFR Part 11 standards. Without these, even great equipment won’t meet regulations.
Space and Layout
Space can be tight. A good filling line fits the facility’s size and layout without losing safety or quality. More companies now prefer modular systems that are easier to install, move, and upgrade.
In the end, the goal is balance—where product, process, equipment, and rules all work well together. When done right, this means reliable production, sterility, and patient safety every time.
Also, when choosing equipment for accurate dosing in injectable lines, it’s important to include air filters and strict cleaning routines to keep things sterile. Monitoring aseptic filling processes is key to maintain quality and meet standards from groups like the Center for Biologics Evaluation and Research (CBER).
8. Regulatory Guidance
In the gleaming corridors of a sterile manufacturing facility, the hum of precision-engineered machines is ever-present—but even louder is the silent pressure of compliance. For every injectable filled, every vial sealed, and every lot released, there’s an invisible thread tying it all back to global regulatory frameworks.
Sterile injectables aren’t just subject to high standards—they’re shaped by them.
Why Regulation Matters
Imagine a cancer patient receiving an injectable chemotherapy drug. There is no margin for error. Every microliter must be safe, potent, and pure. To protect such trust, regulatory agencies like the US FDA, EMA, and CDSCO have built rigorous frameworks—requiring manufacturers to prove that their facilities, processes, and documentation meet global expectations for sterility, traceability, and quality control.
And unlike other dosage forms, injectables are unforgiving. A contaminated tablet may cause mild discomfort—but a contaminated injectable could cause septic shock.
Key Regulatory Frameworks
1. US FDA – 21 CFR Parts 210 and 211
These current Good Manufacturing Practice (cGMP) guidelines govern all drug manufacturing in the United States. They mandate:
- Control over equipment, personnel hygiene, and records
- Validation of sterilization processes
- Environmental monitoring of cleanrooms
- Full traceability of every filled vial
2. EU GMP Annex 1
This is the gold standard for sterile manufacturing in Europe. The updated Annex 1 (2022) introduces:
- Enhanced contamination control strategies (CCS)
- Use of barrier technologies like RABS and isolators
- Strict environmental classifications for cleanrooms
- Focus on visual inspection standards
3. WHO GMP & PIC/S Guidelines
Used in many countries across Asia, Africa, and Latin America, these align closely with EU standards. They emphasize risk-based approaches and harmonized sterilization protocols.
4. CDSCO (India)
India’s Central Drugs Standard Control Organisation adopts a hybrid of WHO GMP and indigenous requirements. It now emphasizes:
- Validation and revalidation of aseptic processes
- Dedicated facility requirements for beta-lactam and cytotoxic injectables
- Data integrity in manufacturing records
What This Means for Manufacturers
Compliance isn’t a checklist—it’s a culture. From gowning procedures and particle monitoring to electronic batch recording, sterile injectable manufacturers must think like regulators at every step.
Some of the operational must-haves include:
- Media fill simulations to validate aseptic operations
- Sterility and endotoxin testing of every batch
- Real-time environmental data capture using LIMS and SCADA
- Comprehensive SOPs and training to minimize human error
And in the age of digital transformation, regulators are raising the bar further—insisting on 21 CFR Part 11-compliant electronic systems, traceable audit trails, and AI-assisted inspection systems that reduce subjectivity.
Final Dose of Insight
Regulatory guidance is not a barrier—it’s a backbone. It ensures that when a child gets vaccinated in a rural clinic or a patient receives an emergency injection in a crowded ER, they’re receiving not just medicine—but certainty.
And for manufacturers, following the rules isn’t just about avoiding warning letters—it’s about honoring a global pact of safety, science, and care.
10. Market Trends & Innovation
Injectable manufacturing is entering a renaissance—a time where the lines between pharmaceutical engineering, digital intelligence, and sustainable innovation are blurring. What once was a static, cleanroom-bound process is now a living, learning system adapting to new demands, new risks, and new possibilities.
1. Rise of Prefilled Syringes & RTU Containers
The global injectable market is rapidly shifting toward prefilled syringes (PFS) and ready-to-use (RTU) formats. These innovations eliminate manual preparation, reduce contamination risk, and enhance patient compliance. Pharmaceutical companies are retrofitting their traditional lines with compact, robotic-compatible modules to process RTU vials and syringes delivered in nested tubs.
2. Gloveless Robotics & Isolator Technology
Companies like Groninger and Syntegon are leading a revolution in robotic aseptic filling. The rise of gloveless isolators with robotic arms has made human intervention nearly obsolete in Class A zones. These systems enhance sterility assurance and align with the EU GMP Annex 1 emphasis on minimizing interventions.
3. Modular & Scalable Systems
The pandemic era forced pharmaceutical companies to rethink flexibility. As a result, modular, skid-based, and plug-and-play systems are becoming mainstream. Optima and IMA are pioneering fill-finish platforms that can be rapidly deployed, scaled, or relocated, making production agile and future-ready.
4. Surge in Single-Use Technologies
To reduce cleaning validation efforts and cross-contamination, single-use systems (SUS)—tubing, bags, manifolds, and filters—are replacing traditional stainless-steel pipelines. They are now standard in biotech and CDMO facilities dealing with multi-product pipelines.
5. Smart Pharma: AI & Digital Twins
AI-driven analytics and digital twins of filling lines are transforming preventive maintenance and real-time optimization. Operators can now visualize process flow, simulate cycle changes, and identify deviations before they occur—bringing predictive control into sterile operations. Our deep dive on digital twins in the pharma sector explores how this technology is revolutionizing cleanroom intelligence.
6. Sustainability Mandates
Eco-efficiency is no longer optional. Energy-efficient HVAC systems, low-waste isolators, recyclable packaging, and leaner facility layouts are becoming essential for regulatory and investor expectations. Sustainability is now deeply embedded in the design philosophy of modern fill-finish lines. Even in semi-solid production, initiatives like sustainable cream manufacturing reflect the industry’s shift towards greener operations.
The future of injectable manufacturing is smarter, faster, and safer. It’s being written by a new generation of machines, algorithms, and quality strategies that aren’t just meeting today’s needs—but anticipating tomorrow’s.
11. Summary & Key Takeaways
As the demand for precision medicine, vaccines, and complex biologics rises, injectable manufacturing is evolving from traditional sterile processes into a dynamic ecosystem driven by automation, digital intelligence, and quality by design.
From understanding the critical nature of injectable formulations to choosing the right filling lines and keeping up with evolving regulations—every step in the journey matters. The industry is moving beyond basic compliance into innovation-led operations that prioritize patient safety, operational agility, and environmental stewardship.
Key Takeaways:
- Injectables are high-risk, high-impact therapies that require 100% bioavailability and sterility. Their manufacturing demands precision and tight regulatory controls.
- Types of injectables vary widely—solutions, powders, lyophilized drugs, cytotoxics—and each requires a tailored manufacturing and filling approach.
- Sterile manufacturing involves a trifecta of aseptic processing, terminal sterilization, and aseptic fill-finish—each chosen based on the product’s properties.
- Modern filling machines range from high-speed digital systems to specialized vial, ampoule, and powder fillers. Machine selection depends on product type, scale, format, and regulatory needs.
- Manufacturers like Syntegon, IMA Life, Groninger, Optima, and Bausch+Ströbel lead global innovation, while Indian firms like ACG, NKP, Lodha, and Harsiddh bring cost-effective, scalable solutions.
- Compliance with global regulations (FDA, EU GMP, CDSCO, PIC/S) is non-negotiable. Validation, traceability, and audit readiness are core pillars of any injectable plant.
- Industry trends are reshaping operations—RTU containers, robotics, modular systems, AI-powered optimization, and sustainability are no longer future goals—they’re current imperatives.
In a world that increasingly relies on injectable medications for everything from diabetes to pandemics, sterile fill-finish is the final safeguard before a drug reaches the patient. And as this guide shows, every dose is backed by science, technology, regulation—and above all, care.
FAQs (Frequently Asked Questions)
What are injectable preparations and why is their precision important?
Injectable preparations are pharmaceutical formulations designed for direct delivery into the body, bypassing the digestive tract. Their precision is crucial because they deliver medicine directly into the bloodstream or tissues, leaving no room for compromise in dosage or sterility, making them essential tools in emergency and critical care.
What types of parenteral preparations exist and how are they administered?
Parenteral preparations include solutions, suspensions, emulsions, and powders, each chosen based on drug properties and stability. They are administered via various routes such as intravenous (IV) for rapid systemic action, intramuscular (IM) for vaccines and long-acting drugs, subcutaneous (SC) for insulin and biologics, intradermal (ID) for allergy tests, and intraperitoneal (IP) mainly in preclinical settings.
What are the different types of injectables used in pharmaceutical manufacturing?
Pharmaceutical manufacturing produces several injectable types including general injectables like vaccines and antibiotics; cytotoxic injectables used in chemotherapy; freeze-dried (lyophilised) injectables preserving sensitive biologics; liquid injectables which are ready-to-use; powder injectables that enhance stability; beta-lactam injectables derived from penicillin; and non-beta-lactam alternatives addressing antibiotic resistance.
How is sterility ensured in the manufacturing process of injectables?
Sterility in injectable manufacturing is maintained through aseptic manufacturing—ideal for heat-sensitive biologics—and terminal sterilization used for stable small molecules. Techniques include aseptic fill-finish using robotic systems within controlled environments like Restricted Access Barrier Systems (RABS), isolators, and real-time environmental monitoring to prevent contamination at every stage.
What infrastructure supports injectable filling lines to ensure accuracy and safety?
Injectable filling lines operate with a focus on accuracy, sterility, and efficiency. They incorporate ultrasonic washing machines, sterilization tunnels with controlled dry heat, precise filling units using peristaltic or rotary pumps, stoppering systems to seal containers instantly, capping, inspection, labeling modules, all housed within Class A and B cleanrooms equipped with HEPA-filtered laminar airflow and real-time monitoring systems to maintain an ultra-clean environment.
What types of injectable filling machines are used in sterile pharmaceutical production?
Sterile pharmaceutical production utilizes various injectable filling machines including liquid filling machines equipped with sensors to detect container presence; digital liquid filling machines featuring smart control systems that monitor each vial's fill process; and compact vial filling machines designed for agility and efficiency. These technologies ensure precise dosing while maintaining sterility throughout the filling process.




