by Mrudula Kulkarni
6 minutes
An Overview On Project Management in the Pharmaceutical Industry
Why project management failures in pharma are often silent, and how disciplined execution determines timelines, compliance, and patient access.
In the pharmaceutical industry, projects rarely fail with drama.
They fail quietly.
- A batch release pushed by weeks.
- A clinical milestone that slips without alarm.
- A technology transfer that drags on longer than planned.
- A product launch that enters the market six months too late.
Each looks like an isolated issue.
In reality, each is a project management failure in the pharmaceutical industry.
Unlike many sectors, the pharmaceutical industry does not offer the luxury of a fast recovery. Every delay compounds costs, regulatory exposure, and opportunity loss. More importantly, it affects patient access. That is why project management in the pharmaceutical industry is not merely administrative support; it is a complex and multifaceted process.
Why Project Management Is Different in the Pharmaceutical Industry
Pharmaceutical project management operates under three immovable constraints:
- Regulatory oversight
- Scientific uncertainty
- High cost of failure
A single pharmaceutical project may span 10 to 15 years, transitioning through drug discovery, preclinical research, clinical trials, manufacturing scale-up, regulatory submissions, commercialisation, and lifecycle management. It involves hundreds of stakeholders across geographies, functions, and regulatory jurisdictions.
Industry estimates suggest that bringing a new drug to market costs between USD 1.5 billion and USD 2.5 billion, factoring in attrition. In this environment, pharmaceutical project management becomes the connective tissue that aligns science, systems, quality, and strategy.
This is why successful pharma organisations treat project management as a core leadership capability, not a reporting function. Regulatory compliance is a defining constraint in pharmaceutical projects.
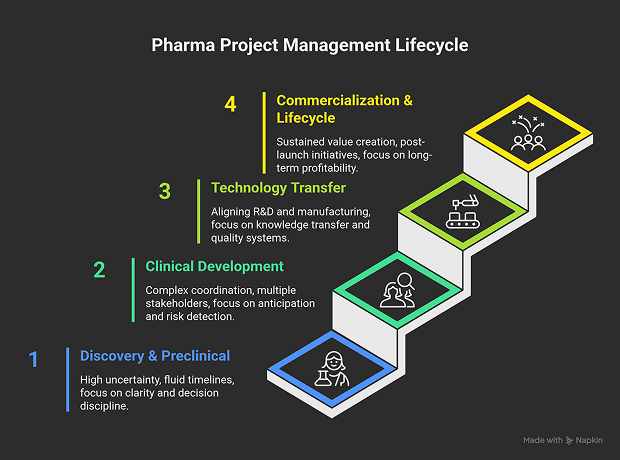
The Lifecycle of Project Management in Pharma
Pharma projects are not linear. They evolve through controlled transitions, each demanding a different project management mindset.
1. Discovery and Preclinical Phase
This is the phase of highest uncertainty.
Timelines remain fluid. Hypotheses change. Data reshapes direction. The role of the pharma project manager here is not to enforce rigid schedules, but to provide clarity, visibility, and decision discipline.
Effective project management at this stage focuses on:
- Defined decision gates
- Clear go/no-go criteria
- Strategic resource prioritization
- Early risk identification
Strong project leadership ensures that scientific curiosity does not drift into unfocused experimentation. Drug discovery project management focuses on decision gates, risk identification, and clear go/no-go criteria.
2. Clinical Development Phase
Once a molecule enters clinical trials, complexity multiplies.
- Multiple sites.
- Multiple CROs.
- Multiple regulators.
- Multiple timelines that must converge.
Clinical project management in the pharmaceutical industry involves synchronising clinical operations, data management, pharmacovigilance, regulatory affairs, and supply chain readiness. A delay in patient recruitment or a protocol amendment can have a ripple effect that spans years.
At this stage, project management shifts from execution to anticipation. The most effective pharma project managers detect risks before they surface in reports.
3. Technology Transfer and Manufacturing Scale-Up
This is where many pharmaceutical projects stumble.
Moving from lab-scale success to commercial manufacturing is not a handover. Scale-up in pharma manufacturing requires validated processes and robust project governance. It is a translation of intent, assumptions, and controls.
Project management during technology transfer must align:
- R&D expectations
- Manufacturing capabilities
- Quality systems
- Regulatory commitments
Failures here are rarely technical alone. They stem from incomplete knowledge transfer, undocumented learning, or misaligned ownership. Effective project management in pharmaceutical manufacturing helps prevent these gaps from becoming compliance risks.
Technology transfer in pharma demands rigorous knowledge capture and clear ownership across functions.
4. Commercialization and Lifecycle Management
Project management does not end at product launch.
Post-launch variations, market expansion, formulation improvements, regulatory updates, and cost optimization initiatives all function as structured projects. In mature pharma organizations, lifecycle project management often determines long-term profitability more than the initial launch itself.
Here, project management becomes a discipline of sustained value creation, not speed.
The Role of a Pharmaceutical Project Manager
A pharmaceutical project manager is not a task tracker.
They are:
- Translators between science and business
- Integrators of cross-functional priorities
- Custodians of timelines without compromising quality
What makes this role unique is the need to understand enough science to ask meaningful questions, sufficient regulation to anticipate compliance risks, and sufficient business context to prioritise decisions.
The most effective pharma project managers consistently:
- Create clarity within complexity
- Enable timely decision-making
- Protect long-term value over short-term acceleration
Regulatory Reality and Project Discipline
In the pharmaceutical industry, compliance is not optional.
Every pharmaceutical project operates within strict regulatory frameworks, including Good Manufacturing Pra,ctices (GMP), Good Clinical Practices (GCP), Good Distribution Practices (GDP), and ICH guidelines, as well as country-specific regulatory requirements, all of which demand rigorous documentation, traceability, and compliance at every stage of the project lifecycle.
This means pharma project management plans must be audit-ready. Documentation, traceability, change control, and deviation management are not parallel activities. They are integral to execution.
Unlike industries where iteration is encouraged freely, pharmaceutical project management strikes a balance between controlled change and continuous improvement.
Project Management Tools and Methodologies in Pharma
Traditional waterfall and stage-gate models continue to dominate regulated pharmaceutical activities. However, the industry is gradually adopting hybrid approaches.
Common methodologies include:
- Stage-gate models for R&D and clinical development
- Agile principles for digital health, analytics, and data systems
- Lean project management for manufacturing and operational excellence
The shift is not about abandoning structure. It is about applying flexibility where science allows and discipline where regulation demands. Digital transformation in pharma is bringing integrated dashboards, predictive risk tools, and real-time visibility to projects.
The Human Side of Pharmaceutical Project Management
Behind every project plan are people.
- Scientists navigating uncertainty.
- Operators balancing throughput and quality.
- Quality professionals holding the line when pressure rises.
Effective project management in the pharmaceutical industry goes far beyond managing timelines and deliverables. It actively ensures stakeholder alignment across functions, resolves conflicts before they escalate, creates psychological safety for teams working under regulatory and commercial pressure, and mitigates decision fatigue by providing clarity, structure, and well-timed governance throughout the project lifecycle.
A well-managed pharma project does not remove pressure. It channels its productivity.
Common Challenges in Pharma Project Management
Despite maturity, several challenges persist across the industry:
- Functional silos
- Late involvement of manufacturing or quality teams
- Over-optimistic timelines
- Poor risk visibility
- Weak knowledge transfer
Most pharmaceutical project failures are not caused by a lack of expertise. They arise from misaligned expectations and fragmented execution.
120260114112318.png)
The Future of Project Management in the Pharmaceutical Industry
The next decade will redefine the field of pharmaceutical project management.
Digital platforms, AI-driven forecasting, integrated dashboards, and real-time risk visibility will improve execution. But technology alone will not fix broken project cultures.
The future belongs to pharmaceutical organizations that intentionally build project literacy across functions, treat project management as a form of leadership development rather than a back-end coordination role, and embed continuous learning directly into their execution models, ensuring that every project strengthens not just outcomes, but organizational capability.
In the pharmaceutical industry, how projects are managed increasingly determines what can be innovated.
Final Thoughts
Project management in the pharmaceutical industry is not about moving faster.
It is about moving right.
- Right decisions.
- Right sequencing.
- Right balance between urgency and responsibility.
In an industry where lives are affected long after project closure, effective project management becomes an ethical obligation, not merely an operational skill.
And that is what makes it one of the most demanding and meaningful disciplines in modern industry.
FAQs
1. Why is project management critical in the pharmaceutical industry?
Because pharmaceutical projects involve long timelines, high costs, regulatory scrutiny, and patient safety, effective project management ensures alignment, risk mitigation, and disciplined execution across complex value chains.
2. How is pharma project management different from other industries?
Pharma project management must operate under strict regulatory frameworks, manage scientific uncertainty, and maintain compliance at every stage, making it more structured and risk-sensitive than most industries.
3. What skills are essential for a pharmaceutical project manager?
Key skills include cross-functional communication, regulatory understanding, risk management, decision facilitation, and the ability to balance scientific, operational, and commercial priorities.
4. Can agile project management be used in pharma?
Yes, selectively. Agile principles are increasingly used in digital, data, and process improvement initiatives, while regulated activities continue to rely on structured, stage-gated approaches.
5. What is the most common reason pharmaceutical projects fail?
Most failures occur due to misalignment rather than lack of expertise. Poor communication, late stakeholder involvement, unrealistic timelines, and weak knowledge transfer are the most common causes.