Key Takeaways
- Blister packaging is essential in pharma for product protection, accurate dosing, and patient compliance.
- Two main types dominate: thermoformed (e.g., PVC-based) and cold-formed (Alu-Alu), each with specific benefits and use cases.
- Material selection PVC, PVDC, PCTFE, COC, aluminum foils) significantly impacts barrier performance, cost, and sustainability.
- The packaging process includes forming, filling, sealing, coding, and final packaging—with increasing automation and Industry 4.0 integration.
- tablets, capsules, oral thin films, patches, and even diagnostic strips,Blister packs are widely used for ensuring product integrity.
- Compliance with global regulations (like FDA 21 CFR, EU GMP, CDSCO) and rigorous quality testing are non-negotiable.
- high protection and traceability, While blister packs offer they come with limitations such as environmental impact and tooling complexity.
I. Executive Summary
Last Updated: May 2025
Disclaimer: This guide is intended for educational purposes only. It does not replace professional or regulatory consultation. Always follow current GMP and regional compliance guidelines.
Blister packaging plays a vital role in the pharmaceutical supply chain, offering a reliable and efficient method for packaging solid dosage forms such as tablets and capsules. It combines product protection, dosage accuracy, regulatory compliance, and user convenience into a single, scalable packaging format.
This guide delves deep into every aspect of blister packaging — from core material science and manufacturing processes to regulatory frameworks and future innovations. For foundational knowledge, explore our comprehensive blister packaging guide.
With a focus on thermoform and cold-form packaging technologies, the guide offers technical insights, practical applications, and expert recommendations for selecting the right packaging solution based on product sensitivity, market needs, and operational efficiency.
Whether you are a packaging engineer, pharma manufacturer, QA professional, or regulatory officer, this guide will provide the foundational and advanced knowledge necessary to make informed decisions around blister packaging strategy in both regulated and emerging markets.
II. Introduction
Blister packaging is a specialized unit-dose packaging format widely adopted in the pharmaceutical industry for its ability to preserve drug integrity, ensure precise dosing, and improve patient compliance.
The process involves thermoforming or cold-forming a base film—typically composed of plastic or aluminum laminate—into cavities that house individual tablets or capsules. These cavities are then sealed with a lidding material, usually aluminum foil, to enclose and protect each dose.
Unlike bulk packaging in bottles, blister packaging provides individualized containment for every unit, thereby enhancing protection from environmental factors and enabling a more controlled patient experience. It is particularly useful in scenarios requiring accurate monitoring of patient adherence, such as in clinical trials, geriatric care, and chronic disease treatment.
The rising complexity of pharmaceutical formulations, global supply chain demands, and stringent regulatory requirements have collectively contributed to the increasing adoption of blister packaging across branded, generic, and over-the-counter (OTC) drug segments.
Want to dive deeper?
Don't miss our expert-curated guide on Blister Packaging in Pharmaceuticals.
Primary Objectives of Blister Packaging:
- Environmental Protection: Shields medications from exposure to moisture, oxygen, light, and contaminants, which can compromise drug stability and efficacy.
- Tamper Evidence: Ensures product integrity through visibly secure packaging, essential for consumer safety and regulatory compliance.
- Patient-Centric Dosing: Supports unit-dose dispensing and compliance through calendar packs or labeled cavities, minimizing dosing errors.
- Portability and Convenience: Lightweight and compact, blister packs are easier to carry, store, and use, particularly for elderly and traveling patients.
- Inventory Management and Trackability: Facilitates better inventory control and traceability throughout the supply chain via serialization and barcoding options.
As the pharmaceutical industry advances toward more personalized and sustainable drug delivery methods, blister packaging stands out as a proven, adaptable, and evolving solution for both manufacturers and end-users.
III. Types of Blister Packaging
A. Thermoform Blister Packaging
Thermoform blister packaging is the most common type used in the pharmaceutical industry due to its cost-effectiveness and production speed. The process involves heating a plastic forming film, such as PVC or its coated variants, until it becomes pliable. This softened film is then shaped into cavities using a mechanical press or vacuum forming. After the cavities are cooled to retain shape, they are filled with tablets or capsules and sealed with aluminum foil.
- Process
- Heating of forming film
- Cavity formation using pressure or vacuum
- Product placement
- Sealing with lidding foil
- Materials:
- PVC (basic), PVC/PVDC (improved barrier), PVC/PE/PVDC (multi-layer for high barrier)
- Advantages:
- Transparent—helps in visual inspection
- Lightweight and easy to handle
- Low material and tooling costs
- Fast forming cycle times
- Limitations:
- Provides only moderate barrier protection (unless coated)
- Not suitable for highly sensitive formulations
B. Cold Form Blister Packaging (Alu-Alu)
Cold forming is used when a high level of protection is required, especially for moisture or light-sensitive drugs. In this method, an aluminum-based laminate is pressed into cavities without applying heat. The laminate typically consists of three layers: oriented polyamide (OPA), aluminum foil, and PVC. This creates an opaque and highly protective blister.
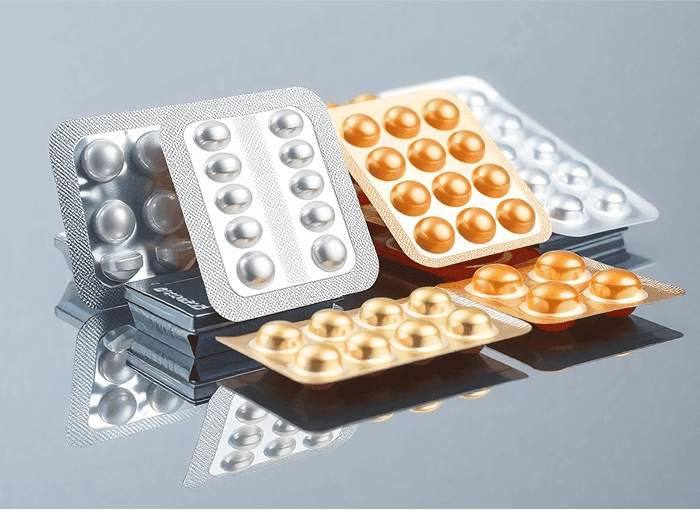
- Process
- Mechanical pressing of Alu-laminate into cavities
- Filling with product
- Sealing with aluminum foil (usually with a heat seal coating)
- Structure:
- OPA/Aluminum/PVC laminate
- Advantages:
- Excellent barrier properties to moisture, oxygen, and light
- Ideal for highly sensitive drugs
- Longer shelf life for medications
- Limitations:
- Opaque—no product visibility
- Slower production speed than thermoforming
- Larger packaging footprint
- Higher material cost and tooling complexity
Thermoform vs Cold Form Blister Packs: Which One Should You Use?
Read MoreCompare strengths, weaknesses, and applications in our in-depth analysis on thermoform vs cold form blister packaging.
C. Specialty Formats
Beyond standard thermoform and cold-form packs, manufacturers have developed customized blister formats to meet specific therapeutic, regulatory, and patient needs.
- Peel-Push Packs: Feature a lidding that can be peeled back before pushing out the dose—helpful for patients with limited hand strength.

Peel-Push Packs
- Peelable Packs: Designed for high-barrier requirements where pushing might damage the drug (e.g., soft gels).
Peelable Packs
- Calendar Packs: Blisters labeled by day or time of administration to support dosing compliance and improve adherence, especially in chronic therapy.
Calendar Packs
- Child-Resistant Packs: Require more force or a specific sequence to open, preventing accidental access by children. Learn what makes a blister child-safe in our write-up on child-resistant blister packaging.
Child-Resistant Packs
- Customized Marketing Formats: Branded packs designed for promotional visibility, enhanced user experience, and product differentiation.

Customized Marketing Formats
These specialty formats are often integrated with high-barrier films and advanced sealing techniques, striking a balance between functionality, safety, compliance, and marketing goals.
IV. Material Science Behind Blister Packaging
A. Forming Materials
Key Considerations When Choosing Forming Materials:
- Moisture & Gas Sensitivity: High barrier materials are essential for hygroscopic or oxidizable drugs.
- Machinability: Materials like PVC are easier and faster to form, reducing cycle times..
- Cost vs. Protection Trade-off: Alu-Alu and Aclar provide superior protection but at a higher material and tooling cost.
- Appearance & Branding: Transparent options like PVC or Aclar allow visual verification of product integrity..
B. Lidding Materials
Lidding materials are crucial for sealing the formed blister cavity and maintaining package integrity until the point of use. The primary function is to protect the product while allowing for ease of access by the patient or healthcare provider.
- Aluminum Foil:
- Standard choice for lidding in most blister applications.
- Excellent barrier to moisture, oxygen, UV, and light.
- Can be printed for branding, regulatory, and usage instructions.
- Push-through and peel-push variants available depending on the sealant type.
- Paper/Foil Laminates:
- Used where printing quality and aesthetics are prioritized.
- Slightly less barrier strength but excellent for unit-dose OTC products and sample packs.
- Sealants (Heat-Seal Coatings):
- Applied to the underside of lidding foil to bond with the forming film.
- Must be compatible with forming substrate (PVC, PVDC, Alu, etc.).
- Available in peelable or non-peelable formats depending on application needs.
Key Considerations When Choosing Forming Materials:
- PVC-Free Films: Sustainable alternatives like COC (Cyclic Olefin Copolymer) or PLA (Polylactic Acid).
- Recyclable Foils & Lidding: Materials designed for easier separation and recycling post-consumption.
- Anti-counterfeit Coatings: Integration of holograms, security inks, and invisible UV patterns for authentication.
Material selection plays a vital role in balancing performance, compliance, and patient usability. Dive into the science of choosing the right material in our guide on comparing blister packaging materials. The right combination ensures the pharmaceutical product reaches the patient with its intended potency and integrity fully preserved.
V. End-to-End Packaging Process Flow
Blister packaging involves a highly controlled and systematic manufacturing process that ensures pharmaceutical products are securely enclosed, accurately labeled, and compliant with global regulatory standards. Below is a step-by-step explanation of the entire process:
- Unwinding of Base Film
- The process begins by feeding the forming film (PVC, PVDC-coated, or Alu-laminate) from a large roll through a film unwinding station.
- Tension control systems ensure smooth and wrinkle-free feed into the forming zone.
- Heating & Forming (or Cold Forming)
- For thermoforming: The film is heated using contact heaters or infrared elements to make it malleable.
- It is then formed into cavities using mechanical pressure or vacuum in a forming mold.
- For cold forming (Alu-Alu): The laminate is mechanically pressed into cavities at room temperature without heating.
- Filling with Solid Oral Dosage Forms
- Tablets or capsules are filled into the formed cavities through vibratory feeders or pick-and-place robotic systems.
- Fill accuracy is critical to avoid product loss and ensure compliance.
- Vision Inspection for Presence/Absence and Defects
- High-speed cameras and sensors inspect each cavity for correct product presence, alignment, and any foreign particles.
- Defective blisters are automatically rejected.
Blister Leak Detection: A Guide to Vacuum Decay, Dye Ingress, and Other QA Techniques
Read MoreUnderstand how pharma ensures sealing integrity using blister leak detection techniques.
- Sealing with Aluminum Lidding
- A lidding foil—usually printed and coated with a heat-seal layer—is positioned over the filled cavities.
- Heat and pressure are applied using a sealing station to bond the foil to the formed film, ensuring a hermetic seal.
- Sealing parameters (temperature, pressure, time) are validated during process development.
- Embossing/Coding (Batch Number, Expiry Date)
- The sealed web is embossed or inkjet/laser-coded with essential product information such as batch number, manufacturing date, and expiry date.
- This ensures full traceability and regulatory compliance.
- Punching/Cutting into Unit Packs
- The continuous sealed web is cut into individual blister cards using die-cutting tools.
- Edge trimming and shaping may be customized based on customer branding or pack design.
- Cartoning and Serialization for Compliance
- Individual blister cards are inserted into folding cartons, which are then serialized with unique identifiers (barcodes, QR codes).
- Serialisation ensures traceability across the supply chain, helping meet regulations like DSCSA (USA) and EU FMD.
- Cartons are finally packed into shipping cases for distribution.
Serialization in Blister Packaging: How to Achieve Global Traceability
Read MoreThroughout the process, in-line quality checks, data recording systems, and compliance validations are integrated to maintain Good Manufacturing Practices (GMP) and ensure that each unit leaving the production line meets safety and quality standards. Learn how serialization ensures global traceability in serialization in blister packaging.
VI. Machinery & Automation
A. Types of Blister Packaging Machines
Blister packaging machinery is central to ensuring consistent product quality, high throughput, and regulatory compliance. Machines vary by technology, production scale, and application needs. Below is a breakdown of common machine types, their benefits, limitations, and key manufacturers.
1. Rotary Blister Machines

- Working Principle: Continuous motion forming and sealing on a rotary drum.
- Advantages:
- High-speed production (up to 600–1000 blisters per minute)
- Suitable for large-scale commercial manufacturing
- Compact footprint with high output
- Limitations:
- Higher initial cost.
- Limited cavity design flexibility compared to flat forming.
2. Flat Forming Blister Machines
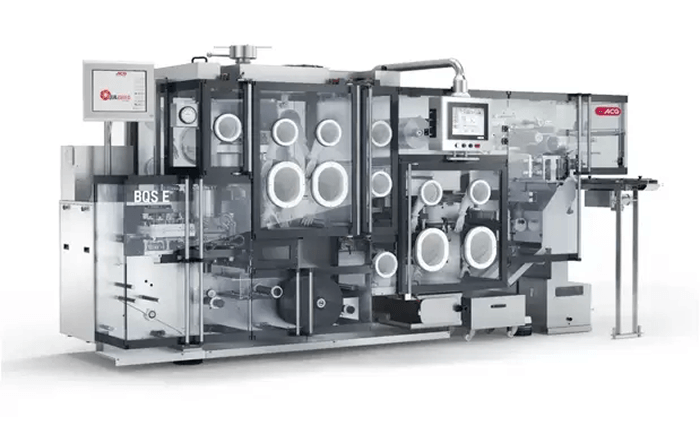
- Working Principle: Uses flat dies to form cavities with pressure and/or vacuum.
- Advantages:
- Allows for more detailed and deeper cavity forming
- Better for fragile or irregular-shaped tablets
- Easier to validate for GMP documentation
- Limitations:
- Lower output than rotary
- Slightly higher cycle time
3. Automatic Blister Packaging Lines

- Working Principle:
- These systems operate using a coordinated series of stations along a continuous or intermittent motion conveyor.
- A forming film is unwound and either thermoformed or cold-formed into cavities.
- Tablets or capsules are inserted into cavities using vibratory feeders or robotic pick-and-place systems.
- Lidding foil is positioned and heat-sealed over the filled cavities.
- The blister strip is coded, cut into unit packs, and passed through inspection systems.
- PLCs (Programmable Logic Controllers) synchronize all stations, while HMIs (Human Machine Interfaces) provide real-time control and monitoring.
- Advantages:
- Fully automated lines reduce labor dependency and enhance production consistency
- High flexibility to switch between blister sizes, formats, and materials
- Better control over GMP documentation and audit trails
- Seamless integration with vision systems and serialization units
- SCADA/MES compatibility enables advanced analytics and traceability
- Limitations:
- Higher capital investment compared to semi-automatic systems
- Complex setup requires skilled operators and technical support
- Maintenance and troubleshooting require downtime and specialized expertise
- Occupies more floor space due to integrated modules
Want to know who's leading pharma packaging?
Check out our quick deep-dive on Top Global Manufacturers shaping the industry.
Read MoreExplore the top innovators in this space in our list of top blister packaging machine manufacturers.
B. Automation & Industry 4.0 Trends
- Digital Twin Technology: Virtual simulation of line performance and OEE tracking.
- MES/ERP Integration: Real-time production data and recipe control
- IoT Connectivity: Predictive maintenance, performance alerts
- Robotic Pick & Place: Enhanced precision in product handling
- AI-based Vision Systems: High-speed product identification, defect detection, and rejection accuracy
C. Selection Factors for Machines
- Type and sensitivity of dosage form
- Required output speed and packaging volume
- Space availability and facility layout
- Regulatory compliance and documentation support
- Selecting the right machine architecture requires balancing speed, flexibility, compliance, and future-proofing for smart manufacturing.
VII. Applications in Pharma
Blister packaging offers flexibility in design, barrier protection, and unit-dose delivery, making it suitable for a wide range of pharmaceutical and healthcare applications. Below are key areas where blister packaging is extensively utilized:
1. Tablets and Capsules (Unit-Dose Packaging)
Blister packaging is most commonly used for solid oral dosage forms such as tablets and capsules. Each unit is sealed in an individual cavity, reducing contamination risk and allowing patients to remove a single dose at a time. This is especially helpful in:
- Chronic care treatments: Helps track daily doses with calendar blisters.
- Hospital settings: Helps track daily doses with calendar blisters.
- Example: Example: Over-the-counter pain relievers like paracetamol and ibuprofen are often blister-packed for consumer convenience and tamper evidence. alerts
2. Oral Thin Films
Blisters are ideal for protecting moisture-sensitive oral thin films (OTFs), such as breath strips or fast-dissolving drug films. These products require high-barrier packaging to maintain integrity and dissolve properly when administered.

- Example: Blister-packed sublingual strips used for rapid drug delivery in pediatric or geriatric patients.
3. Transdermal Patches
Transdermal patches, which deliver medication through the skin over an extended period, require packaging that preserves adhesive strength and active ingredient stability.
- Blister packaging prevents oxidation and moisture ingress while maintaining the patch's flat form and ease of use.
- Example: Nicotine patches for smoking cessation or hormone replacement therapy are often blister-packed.
4. Emerging Use: Injectables in Special Format Blisters
Although injectables are typically stored in vials or ampoules, recent innovations have introduced pre-filled syringes and auto-injectors packed in thermoform blisters for:
- Safe transport and usage tracking
- Ease of administration in home settings
- Example: Emergency adrenaline (epinephrine) auto-injectors like EpiPen are sometimes enclosed in thermoform blister packs for safety and protection.
Blister packaging continues to evolve with innovations in drug delivery formats. As the pharmaceutical landscape adopts more patient-centric therapies and self-administered options, blister packaging will remain a preferred solution due to its adaptability, compliance support, and robust protection.
VIII. Quality Assurance & Regulatory Compliance
Ensuring the safety, efficacy, and integrity of pharmaceutical products requires strict adherence to global regulatory standards and robust quality assurance mechanisms throughout the blister packaging process.
A. Global Guidelines
- FDA (21 CFR 211) - U.S. Food and Drug Administration mandates Current Good Manufacturing Practices (cGMPs) for packaging and labeling operations, including controls for environmental protection, tamper evidence, and material traceability.
- EU GMP Annex 15 - This annex outlines qualification and validation protocols for manufacturing processes, equipment, and packaging systems. It emphasizes risk-based approaches and lifecycle validation of blister lines.
- CDSCO (India) - The Central Drugs Standard Control Organization regulates packaging in India under Schedule M. Requirements include appropriate primary packaging materials, labeling rules, and child-resistant packaging when necessary.
- USP <671>, <659> – The United States Pharmacopeia provides performance testing benchmarks:
- <671> Containers – Performance Testing: Includes tests for moisture vapor transmission rate (MVTR), light transmission, and unit-dose container classifications.
- <659> Packaging and Storage Requirements: Describes packaging system definitions and classifications including light-resistant, tight, and well-closed containers.
B. Testing Protocols
1. Leak Testing
Blisters are ideal for protecting moisture-sensitive oral thin films (OTFs), such as breath strips or fast-dissolving drug films. These products require high-barrier packaging to maintain integrity and dissolve properly when administered.
- Helium Leak Test (advanced): Utilized in high-risk or high-value drugs for sub-micron leak detection.
- Vacuum Decay Method: Detects microleaks by monitoring pressure change within a sealed chamber.
- Dye Ingress Test: Uses colored dye to assess seal integrity by observing penetration through faulty seals.
2. Blister Integrity Testing
- Bubble Emission Test: Submerges the blister under vacuum and observes for bubbles indicating leaks.
- Altitude Simulation: Mimics air cargo pressure changes to ensure packaging does not fail in transit.
3. Barrier Testing
- Water Vapor Transmission Rate (WVTR): Evaluates moisture permeability of the forming and lidding materials.
- Oxygen Transmission Rate (OTR): Measures oxygen ingress that can degrade oxygen-sensitive APIs.
4. Other Tests
- Peel Strength: Ensures adequate but user-friendly sealing force.
- Print Durability and Legibility: Verifies coding remains readable throughout the supply chain.
C. Serialization & Traceability
1. 2D Matrix Barcodes
- Required under U.S. DSCSA and EU FMD for secondary packaging.
- Encodes unique product ID, batch number, expiration date, and serial number.
2. RFID Integration
- Enhances supply chain visibility for high-value medications.
- Used in pilot programs for controlled substances and investigational drugs.
3. Compliance with Global Standards
- DSCSA (U.S.): Enforces drug serialization and transaction documentation across the supply chain.
- EU FMD: Requires tamper-evident features and unique identifiers on each prescription medicine pack.
4. Digital Auditing
- Serialization data is often integrated with Manufacturing Execution Systems (MES) or enterprise software to ensure audit trails and compliance.
By aligning blister packaging operations with these regulatory and QA frameworks, pharmaceutical companies can prevent product recalls, build patient trust, and ensure long-term compliance in a globally harmonized supply chain. Get a detailed roadmap in our blister packaging compliance guide.
IX. Advantages of Blister Packaging
Blister packaging offers a comprehensive array of benefits that make it a preferred choice for pharmaceutical manufacturers and regulators worldwide. These advantages span patient safety, regulatory compliance, and operational efficiency.
- Superior Barrier Protection: Blister packs provide excellent protection against external elements like moisture, oxygen, and light. Compared to bottles, they reduce the exposure of unused doses to environmental stress, thereby preserving drug potency over a longer shelf life. Alu-Alu packs, in particular, offer near-zero water vapor transmission rates, which is essential for hygroscopic and light-sensitive drugs. For a deeper technical understanding, refer to our complete guide on Alu-Alu blister packaging.
- Precise Dose Accuracy & Traceability: Each dose is individually sealed, reducing the risk of underdosing or overdosing. This unit-dose format also allows for clear batch-level and dose-level traceability. Regulatory compliance is easier to maintain, especially in case of recalls or pharmacovigilance activities.
- Improved Patient Adherence: Calendar-style or numbered blisters help patients follow prescribed regimens. This is particularly important in chronic therapies, pediatrics, and geriatrics. Clinical data suggests that calendar packs can improve adherence by up to 30%, especially in therapies requiring consistent timing.
- Tamper-Evident and Secure: Any unauthorized access or compromise in sealing is immediately visible, which builds consumer trust and ensures regulatory compliance. Many blister designs are certified as child-resistant or senior-friendly as per international standards.
- AnyIdeal for Clinical Trials and Specialty Markets: In blinded studies, blisters help maintain dose uniformity and labeling clarity. They also allow for small-batch customization in investigational drug trials. Specialty therapies requiring temperature-sensitive or precise delivery (like oral chemotherapy) also benefit from blister-based formats. See how it’s transforming studies in our guide to blister packaging in clinical trials.
- Lower Cross-Contamination Risk: Since each tablet or capsule is individually sealed, there's reduced risk of cross-contamination during distribution, handling, or storage in clinical and hospital settings.
Together, these advantages make blister packaging not just a containment tool, but a powerful enabler of modern, safe, and user-focused pharmaceutical delivery.
PVC, PVDC, and More: Comparing Blister Packaging Materials for Optimal Drug Protection
Read MoreX. Challenges & Limitations
While blister packaging provides numerous benefits, it also comes with certain drawbacks that must be considered by pharmaceutical companies, especially during scale-up, sustainability planning, or cost evaluation stages.
1. Increased Packaging Waste (PVC/Foil Disposal)
Blister packs typically use a combination of materials such as polyvinyl chloride (PVC), polyvinylidene chloride (PVDC), and aluminum foil, which are laminated together. These materials are not easily separable and pose recycling challenges.
- Environmental Impact: Most blister packs end up in landfills or incinerators, contributing to pharmaceutical packaging waste.
- Regulatory Pressures: A large OTC drug manufacturer in the EU had to switch from traditional PVC-based packs to recyclable mono-material films to meet packaging waste reduction targets.
2. Opaque Packaging Limits Visibility (Alu-Alu)
Cold-formed Alu-Alu blister packs offer superior barrier properties but are non-transparent.
- User Experience Impact: Patients cannot visually verify the product, leading to confusion in polypharmacy (multiple medicines taken daily).
- Clinical Settings: Pharmacists may need to open the pack to confirm contents, increasing contamination risk.
- Example: In geriatric care settings, calendar-labeled transparent PVC/PVDC packs are often preferred for better monitoring.
3. Expensive Tooling for Custom Shapes/Sizes
Creating custom blister cavity shapes or non-standard pack configurations requires dedicated forming and sealing tooling.
- Cost Factor: Tooling changes can cost thousands of dollars and require significant downtime for setup.
- Implication for Low-Volume SKUs: Not viable for small batches or pilot batches unless the added value justifies the expense.
4. Slower Production for Cold-Form Lines Cold-forming
Cold-forming is a purely mechanical process without the aid of heat. This results in a slower cycle time compared to thermoforming.
- Output Bottleneck: May reduce overall packaging line efficiency when high-speed delivery is needed.
- Implication for Low-Volume SKUs: Not viable for small batches or pilot batches unless the added value justifies the expense.
- Footprint & Storage: Larger packs and slower output require more warehousing space and handling time.
- Example: A contract packaging organization reported that shifting high-volume generics from Alu-Alu to barrier-coated thermoform film improved throughput by 30% without sacrificing shelf life.
Understanding these limitations helps pharmaceutical companies make strategic choices that balance product stability, production speed, regulatory compliance, and sustainability goals.
XI. Innovations & Future Trends
A. Sustainability Initiatives
Sustainability is a growing priority in pharmaceutical packaging. Traditional blister materials like PVC and aluminum laminates are difficult to recycle due to their multi-layered structure. To address this, manufacturers and material scientists are developing eco-friendly alternatives:
- Output Bottleneck: May reduce overall packaging line efficiency when high-speed delivery is needed.
- Implication for Low-Volume SKUs: Not viable for small batches or pilot batches unless the added value justifies the expense.
- Footprint & Storage: Larger packs and slower output require more warehousing space and handling time.
- Recyclable and Compostable Blister Materials: New mono-material films (e.g., polypropylene-based) are easier to recycle and meet emerging EPR (Extended Producer Responsibility) norms in regions like the EU. Compostable films made from plant-based polymers are also in early adoption stages for select nutraceutical and wellness products.

- PVC-Free Options Gaining Traction: Explore sustainable alternatives in our breakdown of PVC-free blister packaging. Materials like COC (Cyclic Olefin Copolymer), PET, and PLA are being explored for blister forming, offering comparable barrier properties with improved recyclability. Pharmaceutical brands committed to ESG goals are increasingly opting for these solutions.
- Example: Bayer and Sanofi have piloted recyclable blister formats for OTC brands in Europe to align with circular economy goals.
B. Smart Packaging
Smart blister packaging integrates digital technology to improve patient safety, compliance, and product authentication. Discover how this innovation is reshaping the future in our article on smart blister packaging.
- NFC-Enabled Packs: Near-field communication allows patients to tap their smartphones on the blister pack to receive medication reminders, instructions, or batch verification. Pharma brands are exploring NFC as part of digital adherence programs.

- Patient Adherence Sensors: Some smart blisters embed conductive ink or microcircuits that detect when a pill is removed and transmit that data to a mobile app or caregiver platform—ideal for chronic and high-risk therapies.
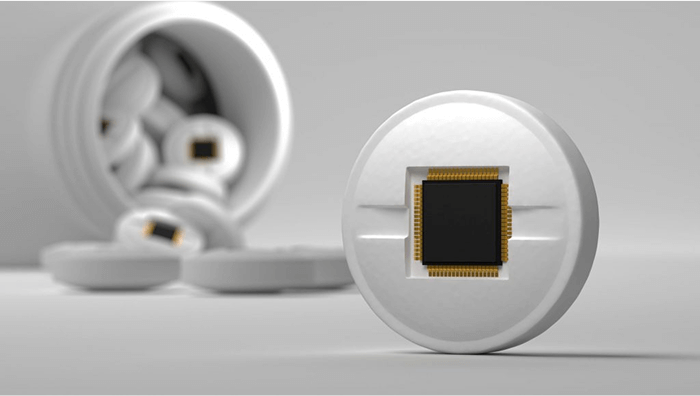
- Digital Pack Tracking (Connected Health): Integrating QR codes or serialized barcodes on blister packs allows real-time track-and-trace across the supply chain. When scanned, these codes can provide origin data, safety instructions, and even AI-based symptom tracking.
- Example: Atrial fibrillation medication programs have used smart blisters with cloud-based adherence logging, resulting in improved treatment continuity.
C. Customization & 3D Printing
As personalized medicine becomes mainstream, packaging must adapt to accommodate patient-specific regimens, delivery formats, and smaller batch sizes.
- Personalized Medicine Packaging: Custom blister formats can align with an individual's treatment schedule, offering labeled packs for days of the week, dosing intervals, or even color-coded medication types. Ideal for oncology, psychiatry, and elderly care.
- Rapid Prototyping for Clinical Studies: 3D-printed blister molds allow rapid design validation and short-run production for trials, allowing faster time-to-market and reduced tooling waste.
- Example: Startups developing niche combination therapies are using 3D-printed cavities to prototype and test multiple packaging configurations within a single week.
These innovations reflect the evolving demands of the pharmaceutical ecosystem—where packaging is no longer just containment, but an interface between patients, providers, regulators, and technologies.
XII. Blister Packaging Design & Selection Checklist
A well-structured checklist can assist packaging engineers, procurement teams, and QA departments in selecting the most appropriate blister format and materials. Below is a downloadable decision-making framework to guide blister packaging selection:
📋 Key Factors to Evaluate:
1. Drug Sensitivity
- Is the product sensitive to moisture, oxygen, or light?
- Does it require Alu-Alu or PVDC-coated films?
- Example: Effervescent tablets require low WVTR materials.
2. Expected Shelf Life
- What is the target shelf life (12/24/36 months)?
- Is the shelf life reduced without cold-chain storage?
- Longer shelf life demands high-barrier forming + lidding.
3. Geographic Market Regulations
- Is the product being exported?
- Are there local sustainability mandates (e.g., PVC-free requirements in Europe)?
- Consider EPR compliance, child-resistance laws, and serialization standards.
4. Dosing Pattern & Patient Type
- Is the drug taken daily, weekly, or PRN (as needed)?
- Is it used by geriatric or pediatric patients (ease of use important)?
- Do you need calendar packs or color-coded blisters?
5. Printing & Branding Needs
- Does the pack need space for usage instructions, QR codes, or branding?
- Are there multilanguage or regulatory text requirements?
- Consider printable lidding and alignment with visual identity.
6. Cost and Supply Chain Constraints
- What is your per-unit cost target?
- Is the packaging line integrated or outsourced?
- Tooling costs for custom formats vs. off-the-shelf designs?
Optional Add-ons:
- Serialization & 2D barcode integration
- Tamper-evident seals
- Sustainable/recyclable material preference
- Smart adherence features (e.g., NFC tags, peel sensors)
Need a quick reference?
Download our handy Pharma Packaging Checklist for key points from this article.
XIII. Glossary
- WVTR: Water Vapor Transmission Rate
- OTR: Oxygen Transmission Rate
- MES: Manufacturing Execution System
- OPA: Oriented Polyamide
- Serialization: Unique identification of drug packages
XIV. References
- U.S. FDA 21 CFR Part 211 – https://www.ecfr.gov/current/title-21/part-211
- EU GMP Annex 15 – https://health.ec.europa.eu
- USP General Chapters <671>, <659>
- WHO Technical Report Series on Packaging
- Romaco & Uhlmann Equipment Brochures
- Published whitepapers from PharmaTech, Fierce Pharma, and SaintyCo
XV. Conclusion
Blister packaging is more than a protective shell—it's a vehicle for safe, compliant, and patient-centric drug delivery. With emerging technologies and shifting regulatory expectations, companies must invest in intelligent, sustainable, and scalable blister solutions to stay future-ready.

Want to dive deeper?
Don't miss our expert-curated guide on Blister Packaging in Pharmaceuticals.